K4000
Kanamycin sulfate from Streptomyces kanamyceticus
Animal Component-free
Synonym(s):
Kanamycin A, Kanamycin sulfate salt
About This Item
Recommended Products
biological source
Streptomyces kanamyceticus
Quality Level
form
powder
potency
≥750 μg per mg (dry basis)
color
white to off-white
solubility
H2O: 50 mg/mL, clear, colorless to faintly yellow
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
Mode of action
protein synthesis | interferes
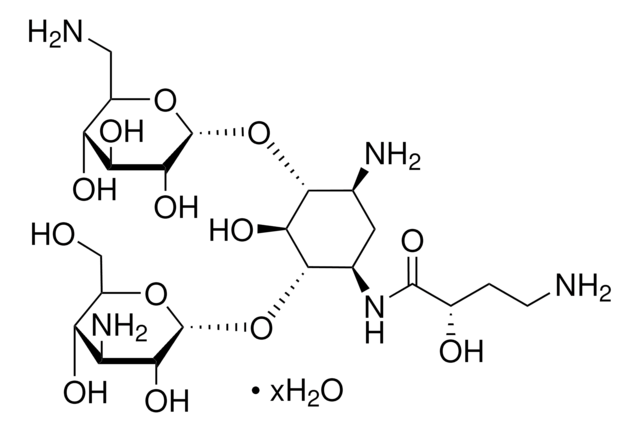
SMILES string
OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H36N4O11.H2O4S/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17;1-5(2,3)4/h4-18,23-29H,1-3,19-22H2;(H2,1,2,3,4)/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-;/m1./s1
InChI key
OOYGSFOGFJDDHP-KMCOLRRFSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- in the research of bacterial antibiotic resistance
- in the research on the effect of different antibiotics on bud regeneration
Biochem/physiol Actions
Mode of Resistance:Aminoglycoside-modifying enzymes (including acetyltransferase, phosphotransferase, nucleotidyltransferase) can alter this antibiotic, preventing its interaction with ribosomes.
Antimicrobial spectrum: Kanamycin sulfate is effective against gram-negative and gram-postiive bacteria, and mycoplasma.
Features and Benefits
Preparation Note
Solutions are stable at 37°C for approximately 5 days. Aqueous stock solutions can be stored at 2-8°C for long term storage.
Storage and Stability
Other Notes
comparable product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service