17773
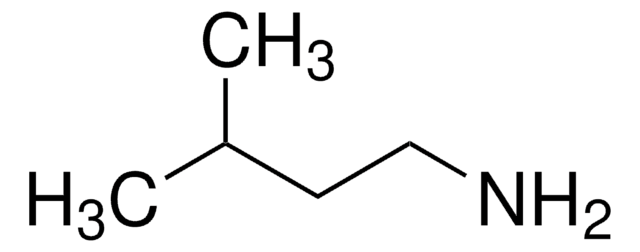
1-Amino-3-methylbutane hydrochloride
puriss., ≥98.0% (TLC)
Synonym(s):
Isopentylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
123.62
Beilstein:
3947083
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
puriss.
Quality Level
Assay
≥98.0% (TLC)
form
solid
mp
65-69 °C
functional group
amine
SMILES string
Cl.CC(C)CCN
InChI
1S/C5H13N.ClH/c1-5(2)3-4-6;/h5H,3-4,6H2,1-2H3;1H
InChI key
HOMVDRDAAUYWKL-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C A Ji et al.
Scientia Sinica. Series B, Chemical, biological, agricultural, medical & earth sciences, 28(11), 1188-1196 (1985-11-01)
Methylisoamylnitrosamine, a carcinogenic N-nitroso compound, has been formed in glucose ammonium nitrate medium containing 150 mg of isoamylamine (a primary amine) inoculated with a common fungus (Fusarium moniliforme Sheldon), to which 400 mg NaNO2 are added after incubation for 7-8
A Tavakkol et al.
Journal of chromatography, 274, 37-44 (1983-05-13)
Columns of Chromosorb 103, Tenax-GC, Amine 220 plus potassium hydroxide on Chromosorb W, and Carbowax 20M plus potassium hydroxide on Chromosorb W were compared for their ability to separate bacterial amines as their free bases in aqueous solution. A 1.52
C Ji et al.
Carcinogenesis, 7(2), 301-303 (1986-02-01)
Nitrosomethylisoamylamine (NMIA), a carcinogenic N-nitroso compound was synthesized from isoamylamine (IAA) in a glucose-ammonium nitrate medium after several days' incubation with fungi and subsequent nitrosation with sodium nitrate. The nitrosamine was not produced by control reactions which lacked either IAA
[Evidence of synthesizing secondary amines from primary amines by fungi--microbiological production of methylisoamylamine and piperidine].
C Ji et al.
Wei sheng wu xue bao = Acta microbiologica Sinica, 26(4), 341-345 (1986-12-01)
S Bu et al.
Biopharmaceutics & drug disposition, 21(4), 157-164 (2001-02-17)
In order to find what types of hepatic cytochrome P450 (CYP) isozymes are involved in the metabolism of 2-(allylthio)pyrazine (2-AP) in rats, enzyme inducers, such as phenobarbital, 3-methylcholanthrene, dexamethasone, or isoniazid, and an enzyme inhibitor, such as SKF 525-A were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service