400036
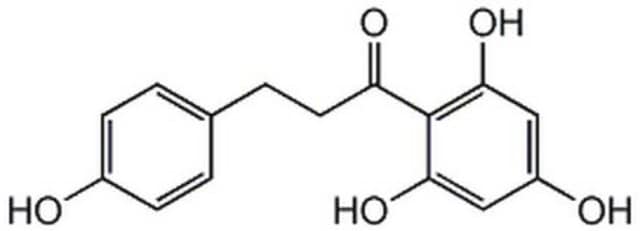
Glucose Transporter Inhibitor IV, WZB117
The Glucose Transporter Inhibitor IV, WZB117 controls the biological activity of Glucose Transporter.
Synonym(s):
Glucose Transporter Inhibitor IV, WZB117, 3-Fluoro-1,2-phenylene bis(3-hydroxybenzoate), GLUT Inhibitor IV, WZB117
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C20H13FO6
CAS Number:
Molecular Weight:
368.31
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated
protect from light
color
white
solubility
ethanol: 100 mg/mL
shipped in
ambient
storage temp.
−20°C
General description
A bis-hydroxybenzoate compound that acts as a fast-acting, irreversible blocker of glucose transport by GLUT1 in red blood cells. Shown to rapidly inhibit glucose transport in cancer cells (IC50 ~ 500 nM in A549 cells) and block proliferation. Its inhibitory effects are more pronounced in hypoxic cancer cells. It binds directly to GLUT1 involving three hydrogen bonds, one each with Asn34, Arg126, and Trp412. Also shown to reduce the levels of GLUT1 protein, intracellular ATP levels, and glycolytic enzymes and increase the level of AMPK in tumor cells. Preferentially induces cell cycle arrest and causes senescence and necrosis in red blood cells and tumor cells (IC50 = 10 µM) over non cancerous cells and synergizes the anti-tumor effects of cisplatin (>Cat. No. 232120) and paclitaxel (>Cat. No. 580555). Also, effectively suppresses tumor growth in human A549 lung cancer grafted nude mice model (10 mg/kg, i.p., daily).
Biochem/physiol Actions
Cell permeable: yes
Primary Target
Glut1
Glut1
Reversible: no
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 2 weeks at -20°C. Unstable in DMSO.
Other Notes
Liu. Y., et al. 2012. Mol. Cancer Ther.11, 1672.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Victoria Sanchez-Martin et al.
Cell chemical biology, 28(11), 1590-1601 (2021-06-25)
Guanine quadruplexes (G4s) are non-canonical nucleic acid structures commonly found in regulatory genomic regions. G4 targeting has emerged as a therapeutic approach in cancer. We have screened naphthalene-diimides (NDIs), a class of G4 ligands, in a cellular model of colorectal
Giulia Salvadori et al.
Cell metabolism, 33(11), 2247-2259 (2021-11-04)
Metastatic tumors remain lethal due to primary/acquired resistance to therapy or cancer stem cell (CSC)-mediated repopulation. We show that a fasting-mimicking diet (FMD) activates starvation escape pathways in triple-negative breast cancer (TNBC) cells, which can be identified and targeted by
Fiona Grimm et al.
The EMBO journal, 43(8), 1545-1569 (2024-03-15)
Adaptation to chronic hypoxia occurs through changes in protein expression, which are controlled by hypoxia-inducible factor 1α (HIF1α) and are necessary for cancer cell survival. However, the mechanisms that enable cancer cells to adapt in early hypoxia, before the HIF1α-mediated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service