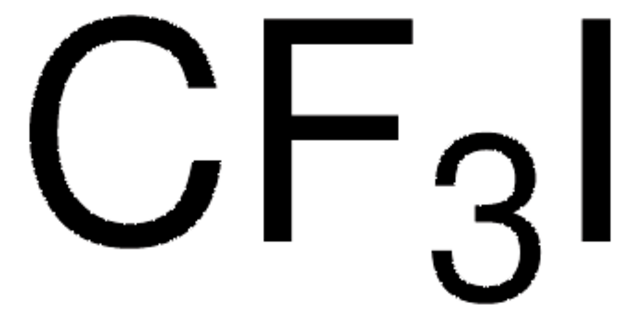777692
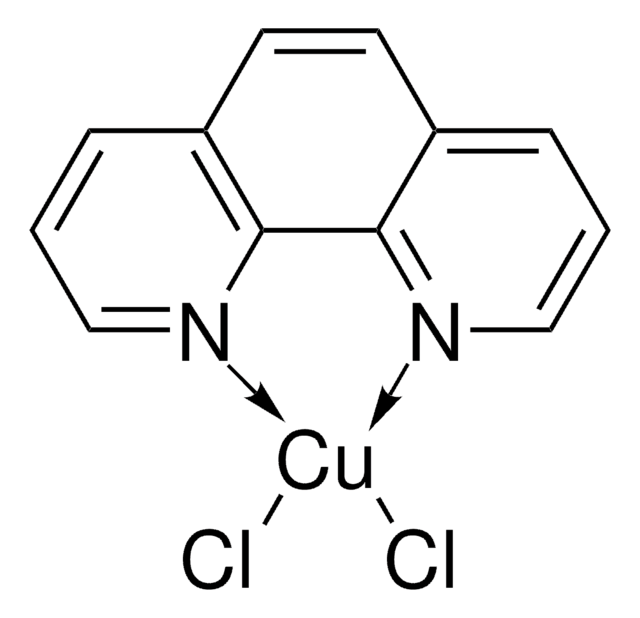
(1,10-Phenanthroline)(trifluoromethyl)copper(I)
90%
Synonym(s):
Trifluoromethylator®
About This Item
Recommended Products
Assay
90%
form
solid
storage temp.
2-8°C
InChI
1S/C12H8N2.CF3.Cu/c1-3-9-5-6-10-4-2-8-14-12(10)11(9)13-7-1;2-1(3)4;/h1-8H;;
InChI key
CUQZQRIIFNWQSJ-UHFFFAOYSA-N
General description
Application
(Phen)Cu-CF3 can be used as a reagent for the trifluoromethylation of aryl iodides. Electron-rich and electron-deficient iodoarenes, as well as sterically-hindered iodoarenes, react in high yield under mild conditions. Electrophilic functional groups, including aldehydes, nitroarenes, ketones, and esters are tolerated. The substrate scope of this reagent is broad and the reagent has wide applicability for trifluoromethylation reactions.
It can also be used in the oxidative trifluoromethylation of organoboron reagents, and terminal alkynes through C-H activation.
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Professor Hartwig's research focuses on the discovery and understanding of new reactions catalyzed by transition metal complexes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service