740136
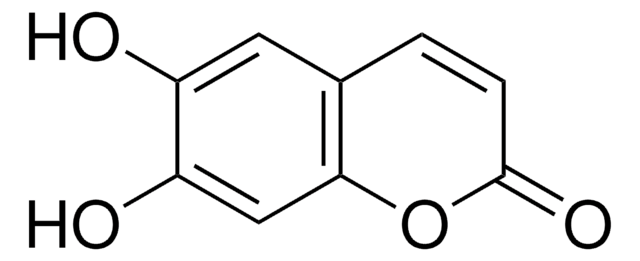
4,7-Dihydroxycoumarin
97%
Synonym(s):
4,7-Dihydroxychromen-4-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H6O4
CAS Number:
Molecular Weight:
178.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
275-280 °C
storage temp.
2-8°C
SMILES string
Oc1ccc2C(O)=CC(=O)Oc2c1
InChI
1S/C9H6O4/c10-5-1-2-6-7(11)4-9(12)13-8(6)3-5/h1-4,10-11H
InChI key
CYSRKZFPSNZSCS-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ilkay Erdogan Orhan et al.
Bioorganic chemistry, 84, 355-362 (2018-12-12)
Coumarins of synthetic or natural origins are an important chemical class exerting diverse pharmacological activities. In the present study, 26 novel O-alkylcoumarin derivatives were synthesized and have been tested at 100 µM for their in vitro inhibitory potential against acetylcholinesterase (AChE)
C Preston Pugh et al.
Archives of biochemistry and biophysics, 564, 244-253 (2014-12-03)
The widely used anticoagulant Coumadin (R/S-warfarin) undergoes oxidation by cytochromes P450 into hydroxywarfarins that subsequently become conjugated for excretion in urine. Hydroxywarfarins may modulate warfarin metabolism transcriptionally or through direct inhibition of cytochromes P450 and thus, UGT action toward hydroxywarfarin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service