All Photos(1)
About This Item
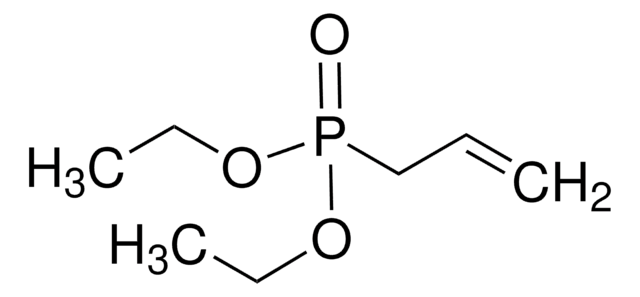
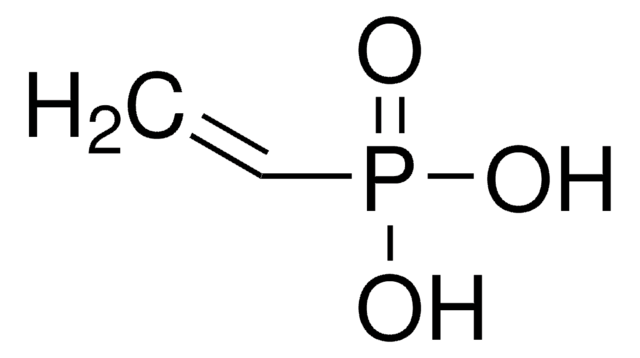
Linear Formula:
(C2H5O)2POCH2C(=CH2)CH3
CAS Number:
Molecular Weight:
192.19
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
reaction suitability
reaction type: C-C Bond Formation
refractive index
n20/D 1.4380 (lit.)
bp
62 °C/0.1 mmHg (lit.)
density
1.013 g/mL at 25 °C (lit.)
functional group
phosphonate
SMILES string
CCOP(=O)(CC(C)=C)OCC
InChI
1S/C8H17O3P/c1-5-10-12(9,11-6-2)7-8(3)4/h3,5-7H2,1-2,4H3
InChI key
QOZGSMHGXZMADD-UHFFFAOYSA-N
General description
Diethyl (2-methylallyl)phosphonate is an organophosphorous compound. It participates in the synthesis of α-aminophosphonate derivatives and azaphosphones. The analgesic/antiinflammatory properties of these derivatives were evaluated.
Application
Diethyl (2-methylallyl)phosphonate can be used as a reagent in the Horner-Wadsworth-Emmons reaction to form conjugated carbon–carbon double bonds.
It can also be used as a reactant for:
It can also be used as a reactant for:
- Enantioselective total synthesis of 10-isocyano-4-cadinene as antifouling agent.
- Regiospecific preparation of 4-oxo-2-alkenylphosphonates (OAP) via silylation followed by Friedel-Crafts acylation and isomerization. OAP can serve as building blocks for the construction of polyethylenic chains.
- The synthesis of azaphosphone as a potent analgesic/anti-inflammatory agents.
Reactant for:
- Enantioselective synthesis of 10-isocyano-4-cadinene and its stereoisomers with antifouling activity
- Preparation of 4-Oxo-2-alkenylphosphonates via silylation followed by regiospecific Friedel-Crafts acylation and isomerization
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I N Smirnova et al.
The journal of physical chemistry. B, 114(50), 16936-16947 (2010-12-01)
The high brilliance of the AILES beamline at the SOLEIL synchrotron facility has been exploited for the study of the gas-phase vibrational spectra of weakly volatile organophosphorous compounds. The propagation of the synchrotron radiation in long path length gas cells
Carbodiimides-key mediators in the synthesis of novel cytotoxic and analgesic/antiinflammatory motifs based on a-amino-, enaminophosphonates, and azaphosphones.
Abdou WM, et al.
Royal Society of Chemistry Advances, 3(5), 1528-1540 (2013)
Keisuke Nishikawa et al.
Organic letters, 12(5), 904-907 (2010-02-06)
The first enantioselective total synthesis of 10-isocyano-4-cadinene, a marine sesquiterpene isolated from nudibranchs of the family Phyllidiidae, was achieved. The cadinene is expected to be a novel nontoxic antifouling agent. In the synthesis, an intermolecular Diels-Alder reaction and a SmI(2)-induced
Lee et al.
The Journal of organic chemistry, 65(13), 4175-4178 (2000-06-24)
Treatment of allylic and vinylic phosphonates with excess LiHMDS, followed by addition of chlorotrimethylsilane, afforded alpha- and gamma- silylated allylic phosphonate mixtures. Without separation, these mixtures underwent the Friedel-Crafts reaction and base-promoted isomerization to give 4-oxo-2-alkenylphosphonates, which can serve as
(-)-Sparteine-mediated stereoselective intramolecular conjugate addition reactions of dienes and enynes
Oestreich M and Hoppe D
Tetrahedron Letters, 40(10), 1881-1884 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service