53588
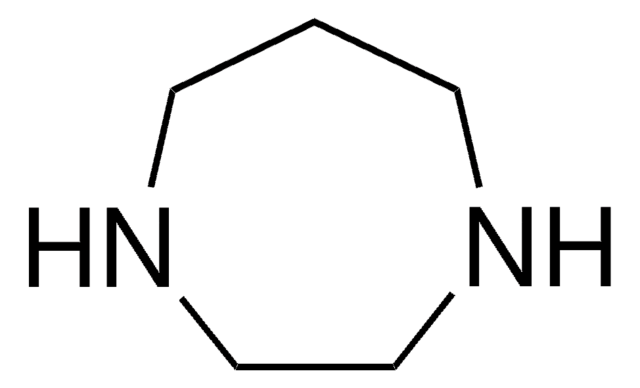
Homopiperazine-1,4-bis(2-ethanesulfonic acid)
Synonym(s):
Hexahydro-1H-1,4-diazepine-1,4-bis(2-ethanesulfonic acid), Homo-PIPES
About This Item
Recommended Products
form
solid
impurities
≤5% water
SMILES string
OS(=O)(=O)CCN1CCCN(CC1)CCS(O)(=O)=O
InChI
1S/C9H20N2O6S2/c12-18(13,14)8-6-10-2-1-3-11(5-4-10)7-9-19(15,16)17/h1-9H2,(H,12,13,14)(H,15,16,17)
InChI key
QZTMPPUJIVQNKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Evaluation of buffers toxicity in plant cells: Homopiperazine-1,4-bis(2-ethanesulfonic acid) was evaluated for its suitability as a buffer in tobacco cell studies at low pH, highlighting its low toxicity and effectiveness in maintaining stable pH environments crucial for plant cell biology experiments (Borgo, 2017).
- Proteomic analysis under stress conditions: This buffer was used in maintaining pH during the proteomic analysis of radicles from seeds treated with aluminum, showcasing its utility in complex biological assays and environmental stress studies (Okekeogbu et al., 2014).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service