466476
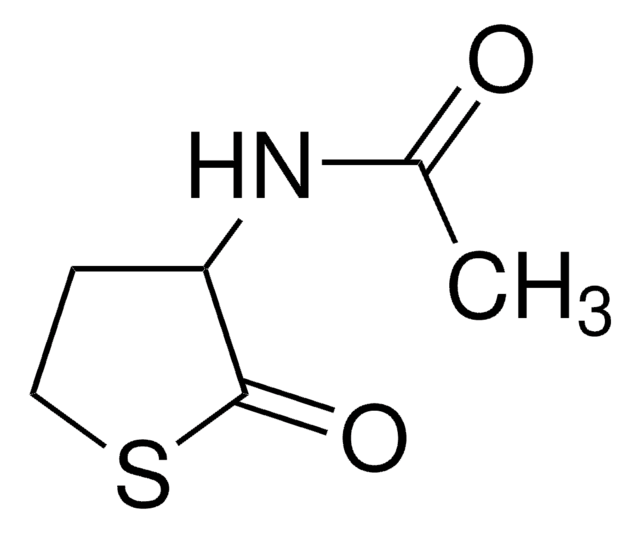
1,3-Bis(methoxycarbonyl)-2-methyl-2-thiopseudourea
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3O2CN=C(SCH3)NHCO2CH3
CAS Number:
Molecular Weight:
206.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
mp
102-105 °C (lit.)
SMILES string
COC(=O)N\C(SC)=N/C(=O)OC
InChI
1S/C6H10N2O4S/c1-11-5(9)7-4(13-3)8-6(10)12-2/h1-3H3,(H,7,8,9,10)
InChI key
KHBXLYPOXVQKJG-UHFFFAOYSA-N
General description
1,3-Bis(methoxycarbonyl)-2-methyl-2-thiopseudourea is a thiourea derivative.
Application
1,3-Bis(methoxycarbonyl)-2-methyl-2-thiopseudourea may be used in the following syntheses:
- methyl (6-methyl-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-2-yl)carbamate
- 2-amino-5-benzyl-6-methyl-3,5-dihydro-4H-pyrrolo[3,2-d]pyrimidin-4-one
- methyl-{5-[(3-hydroxypropyl)thio]-1H-benzo[d]imidazol-2-yl}carbamate (hydroxyalbendazole)
- methyl-{5-[(4-hydroxyphenyl)thio]-1H-benzo[d]imidazol-2-yl} carbamate (hydroxyfenbendazole)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, synthesis, and biological evaluation of classical and nonclassical 2-amino-4-oxo-5-substituted-6-methylpyrrolo [3, 2-d] pyrimidines as dual thymidylate synthase and dihydrofolate
Gangjee A, et al.
Journal of Medicinal Chemistry, 51(1), 68-76 (2007)
Zhexue Wu et al.
Antimicrobial agents and chemotherapy, 57(11), 5448-5456 (2013-08-21)
Albendazole and fenbendazole are broad-spectrum anthelmintics that undergo extensive metabolism to form hydroxyl and sulfoxide metabolites. Although CYP3A and flavin-containing monooxygenase have been implicated in sulfoxide metabolite formation, the enzymes responsible for hydroxyl metabolite formation have not been identified. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service