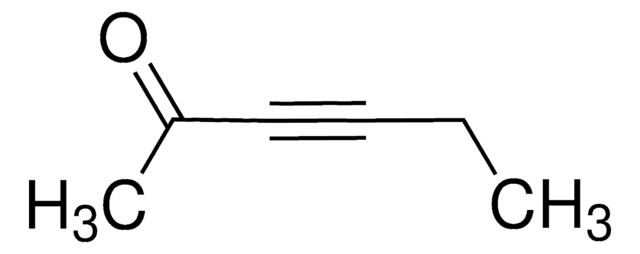All Photos(1)
About This Item
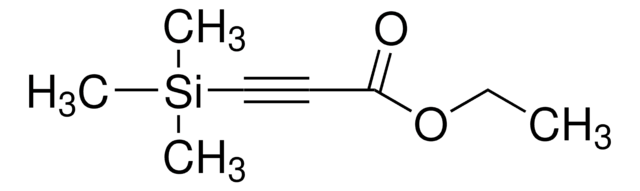
Linear Formula:
(CH3)3SiC≡CCOCH3
CAS Number:
Molecular Weight:
140.26
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.442 (lit.)
bp
156 °C (lit.)
density
0.854 g/mL at 25 °C (lit.)
SMILES string
CC(=O)C#C[Si](C)(C)C
InChI
1S/C7H12OSi/c1-7(8)5-6-9(2,3)4/h1-4H3
InChI key
NQEZDDPEJMKMOS-UHFFFAOYSA-N
General description
4-(Trimethylsilyl)-3-butyn-2-one is a ketone. Its asymmetric bioreduction to enantiopure {(S)-TMSBOL in various hydrophilic ionic liquid (ILs) solvent systems has been reported.
Application
4-(Trimethylsilyl)-3-butyn-2-one (TMSB) has been used to investigate its asymmetric bioreduction to (S)-4-(trimethylsilyl)-3-butyn-2-ol {(S)-TMSBOL} by employing biocompatible water-immiscible ionic liquids (ILs). TMSB may be used for the synthesis of entecavir (BMS-200475).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
82.4 °F - closed cup
Flash Point(C)
28 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Javier Velasco et al.
The Journal of organic chemistry, 78(11), 5482-5491 (2013-05-18)
Entecavir (BMS-200475) was synthesized from 4-trimethylsilyl-3-butyn-2-one and acrolein. The key features of its preparation are: (i) a stereoselective boron-aldol reaction to afford the acyclic carbon skeleton of the methylenecylopentane moiety; (ii) its cyclization by a Cp2TiCl-catalyzed intramolecular radical addition of
Wen-Yong Lou et al.
BMC biotechnology, 9, 90-90 (2009-10-24)
Whole cells are usually employed for biocatalytic reduction reactions to ensure efficient coenzyme regeneration and to avoid problems with enzyme purification and stability. The efficiency of whole cell-catalyzed bioreduction is frequently restricted by pronounced toxicity of substrate and/or product to
Bo-Bo Zhang et al.
PloS one, 7(5), e37641-e37641 (2012-06-05)
Hydrophilic ionic liquids (ILs) were employed as green solvents to construct an IL-containing co-solvent system for improving the asymmetric reduction of 4-(trimethylsilyl)-3-butyn-2-one by immobilized Candida parapsilosis cells. Among 14 hydrophilic ILs examined, 1-(2'-hydroxyl)ethyl-3-methylimidazolium nitrate (C(2)OHMIM·NO(3)) was considered as the most
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service