316539
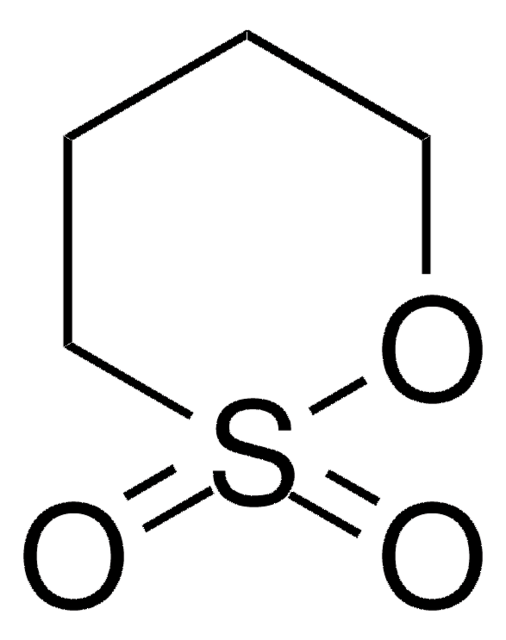
1,8-Naphthosultone
98%
Synonym(s):
1-Naphthol-8-sulfonic acid sultone, 8-Hydroxynaphthalene-1-sulfonic acid sultone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H6O3S
CAS Number:
Molecular Weight:
206.22
Beilstein:
9381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
154-157 °C (dec.) (lit.)
functional group
sulfonic acid
SMILES string
O=S1(=O)Oc2cccc3cccc1c23
InChI
1S/C10H6O3S/c11-14(12)9-6-2-4-7-3-1-5-8(13-14)10(7)9/h1-6H
InChI key
IEIADDVJUYQKAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sébastien Schmitt et al.
Chemical communications (Cambridge, England), 47(41), 11465-11467 (2011-09-29)
Sultones were subject to ring opening by nucleophilic attack with [(18)F]fluoride to afford easily purified (18)F-labelled hydrophilic sulfonated products in high yields. A two-step sequence including radiofluorination and coupling to lysine was then developed from a bis-sultone precursor as a
The derivation of quantitative correlations between skin sensitisation and physio-chemical parameters for alkylating agents, and their application to experimental data for sultones.
D W Roberts et al.
Journal of theoretical biology, 99(4), 807-825 (1982-12-21)
Donald C Craig et al.
Carbohydrate research, 346(6), 854-857 (2011-03-11)
Acetolysis of methyl 5,6-di-O-acetyl-2,3-O-isopropylidene-β-L-gulofuranoside has yielded a sultone, 4-(1,2,5,6-tetra-O-acetyl-β-L-gulofuranos-3-yl)-6-methyl-1,2-oxathiin 2,2-dioxide (2) whose structure was determined by X-ray diffraction. (1)H and (13)C NMR spectral properties of 2 are presented together with a rationale for its formation. Preparation and properties of the
E Meschkat et al.
Chemical research in toxicology, 14(1), 118-126 (2001-02-15)
3-[(13)C]- and 2-[(13)C]hex-1-ene-1,3-sultones (1a and 1b, respectively) and 3-[(13)C]hex-1-ene-1,3-sultone 2a were incubated with human serum albumin in phosphate buffer at pH 8.1. In both cases, the main reaction was a hydrolysis via an S(N) reaction at position 3, but several
DNA adducts of lactones, sultones, acylating agents and acrylic compounds.
J J Solomon
IARC scientific publications, (125)(125), 179-198 (1994-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service