All Photos(1)
About This Item
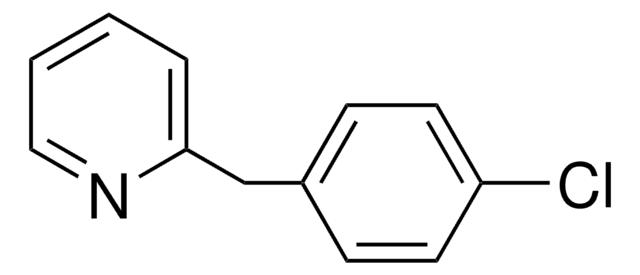
Linear Formula:
ClC6H4OCH2COCl
CAS Number:
Molecular Weight:
205.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.5486 (lit.)
bp
142 °C/17 mmHg (lit.)
mp
18.8 °C (lit.)
density
1.314 g/mL at 25 °C (lit.)
SMILES string
ClC(=O)COc1ccc(Cl)cc1
InChI
1S/C8H6Cl2O2/c9-6-1-3-7(4-2-6)12-5-8(10)11/h1-4H,5H2
InChI key
VRBVHQUSAOKVDH-UHFFFAOYSA-N
Application
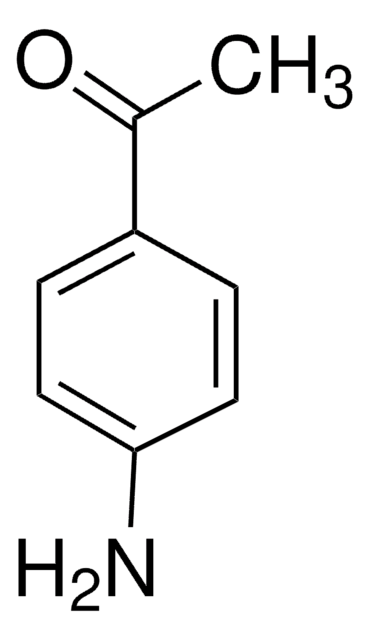
4-Chlorophenoxyacetyl chloride was used in the preparation of:
- substituted acetophenone derivatives
- 2-(4-chlorophenoxyacetylamino)-3-ethoxycarbonyl[2,3-b]quinuclidine
- 2-(4-chlorophenoxy)-N′-[2-(4-chlorophenoxy)acetyl]acetohydrazide monohydrate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ting Chen et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 11), o2829-o2829 (2010-01-01)
In the title compound, C(16)H(14)Cl(2)N(2)O(4)·H(2)O, the hydrazine and water mol-ecules are both located on twofold axes. The C-N-N-C torsion angle is -72.66 (1)° and the dihedral angle between the two benzene rings is 67.33 (1)°. In the crystal, mol-ecules are linked into
Jonathan Rosen et al.
Organic letters, 9(4), 667-669 (2007-01-27)
A direct and efficient method was developed for the preparation of a variety of substituted acetophenone derivatives from readily available arene precursors and acid chlorides. This method has significant generality and affords access to substitution patterns on aryl rings not
Synthesis and properties of 2-amino-3-ethycarbonylthieno [2, 3-b] quinuclidines.
Kaminka ME, et al.
Pharmaceutical Chemistry Journal, 21(8), 568-570 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service