All Photos(1)
About This Item
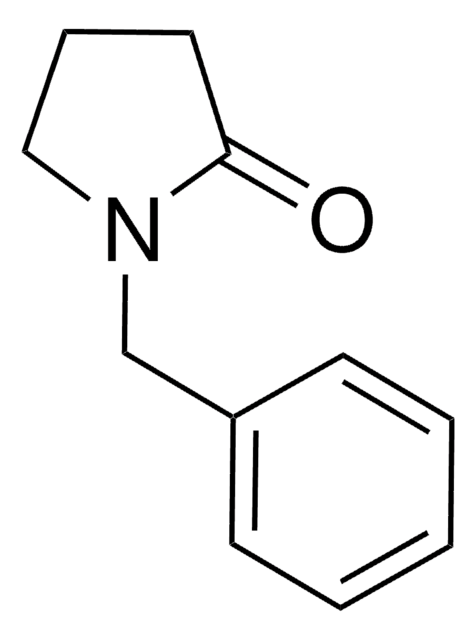
Empirical Formula (Hill Notation):
C11H13NO
CAS Number:
Molecular Weight:
175.23
Beilstein:
1526217
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.539 (lit.)
bp
77 °C/0.01 mmHg (lit.)
density
1.091 g/mL at 25 °C (lit.)
functional group
ketone
phenyl
storage temp.
2-8°C
SMILES string
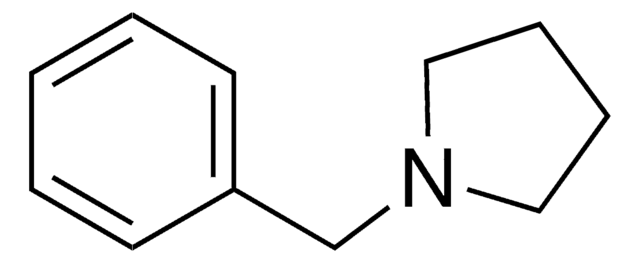
O=C1CCN(C1)Cc2ccccc2
InChI
1S/C11H13NO/c13-11-6-7-12(9-11)8-10-4-2-1-3-5-10/h1-5H,6-9H2
InChI key
DHGMDHQNUNRMIN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
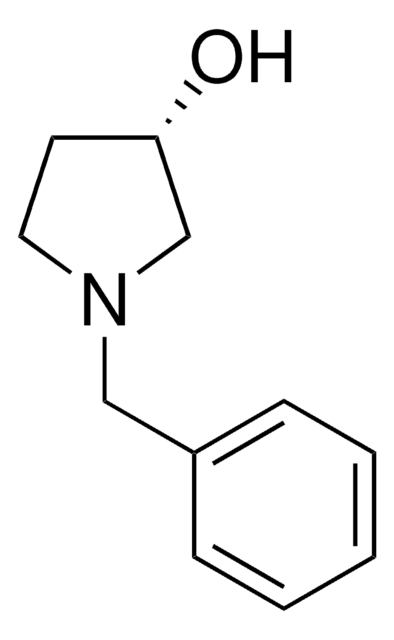
The equilibrium constant for the ketoreductase-catalyzed reduction reaction of 1-benzyl-3-pyrrolidinone has been measured in n-hexane. 1-Benzyl-3-pyrrolidinone on enzymatic asymmetric reduction yields enantiopure 1-benzyl-3-hydroxypyrrolidine, well known intermediate for various drugs.
Application
1-Benzyl-3-pyrrolidinone was used as starting reagent in the synthesis of vinyl triflate. It was used to prepare chiral, alkenyl sulfoximines leading to highly functionalized diazabicycles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Facile synthesis of 3-arylpyrroles by tandem Suzuki-dehydrogenation reaction.
Lee C-W and Chung YJ.
Tetrahedron Letters, 41(18), 3423-3425 (2000)
Synthesis, 2224-2224 (2006)
Practical synthetic process for enantiopure 1-benzyl-3-hydroxypyrrolidine.
Morimoto M and Sakai K.
Tetrahedron Asymmetry, 19(12), 1465-1469 (2008)
A thermodynamic study of ketoreductase-catalyzed reactions 5. Reduction of substituted ketones in n-hexane.
Tewari YB, et al.
The Journal of Chemical Thermodynamics, 40(4), 661-670 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service