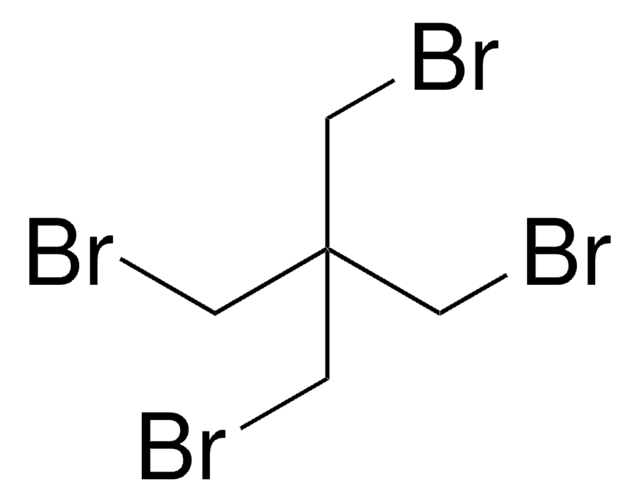
185140
3,3′-Dichloropivalic acid
≥97%
Synonym(s):
2,2-Bis(chloromethyl)propionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C(CH2Cl)2CO2H
CAS Number:
Molecular Weight:
171.02
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
solid
mp
64-66 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
CC(CCl)(CCl)C(O)=O
InChI
1S/C5H8Cl2O2/c1-5(2-6,3-7)4(8)9/h2-3H2,1H3,(H,8,9)
InChI key
DDSPBKFTRPWDLI-UHFFFAOYSA-N
Related Categories
General description
3,3′-Dichloropivalic acid reacts with ethane-1,2-diamine to yield isomeric tetra-amine derivatives, tetra amino carboxylic acid and carboxamidotriamino alcohol.
Application
3,3′-Dichloropivalic acid was used in the synthesis of 3,3′-dichloropivaloyl chloride.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A novel rearrangement reaction conversion of 3-(chloromethyl) azetidin-2-ones to azetidine-3-carboxylic acid esters.
Bartholomew D and Stocks MJ.
Tetrahedron Letters, 32(36), 4795-4798 (1991)
Paul V Bernhardt et al.
Inorganic chemistry, 43(5), 1681-1688 (2004-03-03)
Reaction between ethane-1,2-diamine and 3,3'-dichloropivalic acid results in different, isomeric tetra-amine derivatives, one a tetraamino carboxylic acid and the other a carboxamidotriamino alcohol, depending upon reaction conditions. Intended conversion of the Cu(II) complex of the former to a cyclam-like macrocycle
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service