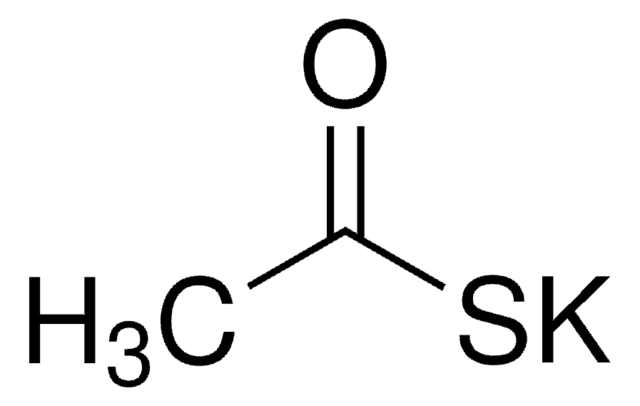
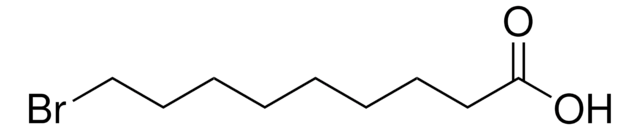
All Photos(3)
About This Item
Linear Formula:
Br(CH2)10COOH
CAS Number:
Molecular Weight:
265.19
Beilstein:
1767205
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
95%
bp
173-174 °C/2 mmHg (lit.)
mp
45-48 °C (lit.)
SMILES string
OC(=O)CCCCCCCCCCBr
InChI
1S/C11H21BrO2/c12-10-8-6-4-2-1-3-5-7-9-11(13)14/h1-10H2,(H,13,14)
InChI key
IUDGNRWYNOEIKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
11-Bromoundecanoic acid reacts with potassium salt of dimethyl hydantoin to yield 4,4-dimethyl hydantoin-undecanoic acid.
Application
11-Bromoundecanoic acid was used in the synthesis of 11-phenoxyundecyl phosphate and 11-hydroxytetradecanoic acid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Revathi V Padmanabhuni et al.
Industrial & engineering chemistry research, 51(14), 5148-5156 (2012-09-04)
The purpose of this study was to demonstrate that the surface of CaCO(3) fillers could be coated with an N-halamine based fatty acid to make the filler surface organophilic and accomplish antibacterial activity simultaneously, rendering the resulting polymer-filler composites antimicrobial.
L L Danilov et al.
Bioorganicheskaia khimiia, 35(3), 431-432 (2009-07-22)
A new scheme of synthesis of 11-phenoxyundecyl phosphate from 11-bromoundecanoic acid was suggested for its ability to react as an acceptor of 2-acetamido-2-deoxy-alpha-D-glucopyranosyl phosphate in a reaction catalyzed by UDP-N-acetylglucosamine : polyprenyl phosphate N-acetylglucosamine phosphotransferase from Salmonella arizona O:59.
I Navarro et al.
Bioorganic & medicinal chemistry, 4(3), 439-443 (1996-03-01)
The synthesis of deuterium labeled 11- and 12-hydroxytetradecanoic acids to study a (11E) desaturase in the moth Spodoptera littoralis is reported. [14,14,14-2H3] 12-hydroxytetradecanoic acid was synthesized in four steps from 11-iodo-1-undecene in 49% overall yield. Deuterium was introduced by reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service