MAK172
Proteasome 20S Activity Assay Kit
sufficient for 100 fluorometric tests
Synonym(s):
20S Proteasome Detection Kit
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
usage
sufficient for 100 fluorometric tests
detection method
fluorometric
relevant disease(s)
endocrinological disorders, diabetes; neurological disorders; cancer
storage temp.
−20°C
Related Categories
General description
Protein degradation occurs most commonly through two major mechanisms, lysosomal degradation and proteasome mediated degradation. Lysosomal degradation occurs through proteolytic enzyme activity and is fairly non-specific. Proteasomal degradation is targeted for a specific protein through the ubiquitination of the target protein. The target protein is typically poly-ubiquitinated and activates the 26S proteasome toward proteolysis. The proteasome 26S is the most common form of the proteasome and is an ATP-dependent proteolytic complex, which contains one 20S (700-kDa) core particle structure and two 19S (700-kDa) regulatory caps.
The proteasome is responsible for the recycling of proteins and maintenance of the balance of protein synthesis and degradation. The proteasome is involved with apoptosis, DNA repair, endocytosis, and cell cycle and has been implicated in disease states such as certain types of cancer, diabetes mellitus, and Alzheimer′s disease among others making it the target for drug discovery investigations.
The proteasome is responsible for the recycling of proteins and maintenance of the balance of protein synthesis and degradation. The proteasome is involved with apoptosis, DNA repair, endocytosis, and cell cycle and has been implicated in disease states such as certain types of cancer, diabetes mellitus, and Alzheimer′s disease among others making it the target for drug discovery investigations.
Features and Benefits
Compatible with high-throughput handling systems.
Suitability
Suitable for measuring the chymotrypsin-like protease activity associated with the proteasome complex either in cultured cells or cell lysates.
Principle
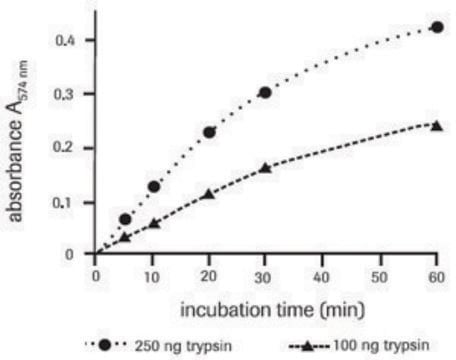
The fluorometric proteasome 20S assay kit is a homogeneous fluorescent assay that measures the chymotrypsin-like protease activity associated with the proteasome complex in cultured cells. This kit uses LLVY-R110 as a fluorogenic indicator for proteasome activities. Cleavage of LLVY-R110 by proteasome generates strongly green fluorescent R110 that is monitored fluorimetrically at 520-530 nm with excitation at 480-500 nm. The assay is robust, and can be readily adapted for high-throughput assays to evaluate the proteasome activities or screen the inhibitors in cultured cells or in solution. The assay can be performed in a convenient 96-well and 384-well fluorescence microtiter-plate format.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Cell: A Molecular Approach (2000)
Javier Quero et al.
Antioxidants (Basel, Switzerland), 10(2) (2021-02-11)
The application of plant extracts for therapeutic purposes has been used in traditional medicine because plants contain bioactive compounds with beneficial properties for health. Currently, the use of these compounds that are rich in polyphenols for the treatment and prevention
Mahamud-Ur Rashid et al.
Journal of virology, 96(5), e0199021-e0199021 (2022-01-13)
Influenza A virus (IAV), an obligatory intracellular parasite, uses host cellular molecules to complete its replication cycle and suppress immune responses. Proteasome subunit alpha type 2 (PSMA2) is a cellular protein highly expressed in IAV-infected human lung epithelial A549 cells.
Shin-Ichi Makino et al.
Journal of the American Society of Nephrology : JASN, 32(3), 597-613 (2021-01-30)
The ubiquitin-proteasome system (UPS) and the autophagy-lysosomal system (APLS) are major intracellular degradation procedures. The importance of the APLS in podocytes is established, but the role of the UPS is not well understood. To investigate the role of the UPS
Inés Mármol et al.
Inorganic chemistry, 59(23), 17732-17745 (2020-11-19)
A series of gold(I) and silver(I) derivatives with N- or S-donor ligands derived from 2-anilinopyridine has been synthesized and characterized. The mononuclear structure of [Au(L1)(PPh3)](TfO) (1a) and [Au(L2)(PPh3)](TfO) (1b) was confirmed by X-ray diffraction studies, as well as the dinuclear
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service