900661
Methoxy poly(ethylene glycol)-b-poly(D,L-lactide)
4k-2.2k
Synonym(s):
mPEG-b-PLA, mPEG-PLA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
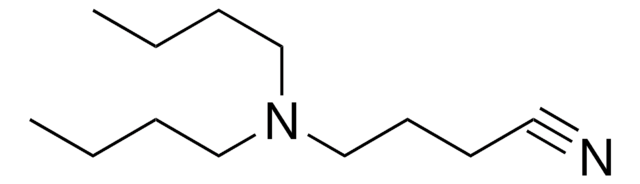
HO[CH(CH3)COO]m[CH2CH2O]nCH3
UNSPSC Code:
12162002
NACRES:
NA.23
Recommended Products
Looking for similar products? Visit Product Comparison Guide
Application
Biocompatible, amphiphilic block copolymer composed of a hydrophilic PEG block and a hydrophobic poly(D,L-lactide) (PLA) block. These materials have been used in control release and nanoparticle formulation for drug encapsulation and delivery applications. Well-defined materials with varying properties can be prepared by controlling the relative length of each polymer block. Hydroxyl termination allows for facile further chemical modification of these materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zahra Daman et al.
Pharmaceutical research, 32(11), 3756-3767 (2015-08-01)
Resistance to gemcitabine in pancreatic cancer (PC) may account for the failure of conventional treatments. Recently, salinomycin (SAL) has been identified as selective inhibitor of cancer stem cells (CSCs). In our study, we aimed to deliver SAL to gemcitabine-resistant PC
Ho-Chul Shin et al.
Journal of controlled release : official journal of the Controlled Release Society, 140(3), 294-300 (2009-05-05)
Current clinical and preclinical anticancer formulations are limited by their use of toxic excipients and stability issues upon combining different drug formulations. We have found that poly(ethylene glycol)-block-poly(d,l lactic acid) (PEG-b-PLA) micelles can deliver multiple poorly water-soluble drugs at clinically
Yiguang Wang et al.
Pharmaceutical research, 27(9), 1861-1868 (2010-06-19)
To develop an efficient tumor vasculature-targeted polymeric micelle delivery system for combretastatin A4 (CA4), a novel antivascular agent. CA4-loaded micelles were prepared from poly (ethylene glycol)-b-poly (d, l-lactide) copolymers. RGD peptides that target integrins alphavbeta3 and alphavbeta5, markers of angiogenic
Yuan Zhang et al.
Pharmaceutical research, 28(5), 1167-1178 (2011-02-23)
Somatostatin analogue octreotide (OCT)-modified PEG-b-PLA micelles were constructed to bind to somatostatin receptors (SSTRs) overexpressed on tumor cells for enhanced intracellular drug delivery and improved therapeutic efficacy for malignant tumors. Copolymers conjugated with octreotide (OCT-PEG₆₀₀₀-b-PLA₅₀₀₀) were synthesized. The fluorescent probe
Rui Su et al.
Cancer cell, 38(1), 79-96 (2020-06-13)
Fat mass and obesity-associated protein (FTO), an RNA N6-methyladenosine (m6A) demethylase, plays oncogenic roles in various cancers, presenting an opportunity for the development of effective targeted therapeutics. Here, we report two potent small-molecule FTO inhibitors that exhibit strong anti-tumor effects
Articles
The development of drugs that target specific locations within the human body remains one of the greatest challenges in biomedicine today.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service