796549
Stahl Aerobic Oxidation TEMPO solution
0.2 M in acetonitrile, Solution for Oxidation of Primary Alcohols
About This Item
Recommended Products
Quality Level
form
liquid
reaction suitability
reagent type: oxidant
concentration
0.2 M in acetonitrile
storage temp.
2-8°C
SMILES string
CN1C=CN=C1.CC2(C)CCCC(C)(C)N2[O].C3(C4=NC=CC=C4)=NC=CC=C3
InChI
1S/C10H8N2.C9H18NO.C4H6N2/c1-3-7-11-9(5-1)10-6-2-4-8-12-10;1-8(2)6-5-7-9(3,4)10(8)11;1-6-3-2-5-4-6/h1-8H;5-7H2,1-4H3;2-4H,1H3
InChI key
BQFURWVGIDXRNB-UHFFFAOYSA-N
General description
Application
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1C
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
35.6 °F
Flash Point(C)
2.0 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Related Content
he Stahl Lab focuses on the development of catalysts and catalytic reactions for selective oxidation of organic molecules, with particular emphasis on aerobic oxidation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
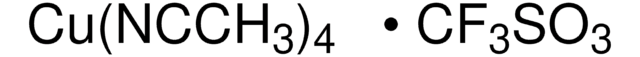
![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)









