All Photos(1)
About This Item
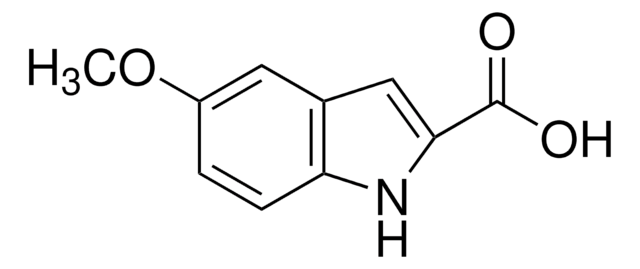
Empirical Formula (Hill Notation):
C9H6FNO2
CAS Number:
Molecular Weight:
179.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
259 °C (dec.) (lit.)
SMILES string
OC(=O)c1cc2cc(F)ccc2[nH]1
InChI
1S/C9H6FNO2/c10-6-1-2-7-5(3-6)4-8(11-7)9(12)13/h1-4,11H,(H,12,13)
InChI key
WTXBRZCVLDTWLP-UHFFFAOYSA-N
General description
5-Fluoroindole-2-carboxylic acid is an antagonist of the glycine site within the NMDA (N-methyl-D-aspartate) receptor complex.
Application
Reactant for the synthesis of:
- Fungicidal agents
- Antitumor agents
- 2,3-dioxygenase (IDO) inhibitors
- Factor Xa inhibitors
- Enantioselective D3 receptor antagonists
- Ligands for hFPRL1 (or ALXR) receptor in inflammation
- Antibacterial agents
- Inhibitors of hepatitis C virus NS3·4A protease
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chung-Yi Wu et al.
Chemistry & biology, 13(3), 261-268 (2006-04-28)
Severe acute respiratory syndrome (SARS) is caused by a newly emerged coronavirus that infected more than 8000 individuals and resulted in more than 800 fatalities in 2003. Currently, there is no effective treatment for this epidemic. SARS-3CL(pro) has been shown
T J Grudt et al.
Molecular pharmacology, 37(4), 477-481 (1990-04-01)
Whole-cell and single-channel patch-clamp recordings from hippocampal neurons in culture have been used to study the receptor channel selectivity of the glutamate analog quisqualate. The dose-response relationship of quisqualate acting at the N-methyl-D-aspartate (NMDA) receptor was measured as that portion
R Kapoor et al.
Clinical and experimental pharmacology & physiology, 25(3-4), 216-219 (1998-05-20)
1. The effects of the specific N-methyl-D-aspartate (NMDA)-glycine site antagonist 5-fluoro indole-2-carboxylic acid (FICA) and NMDA, microinjected into the vasodepressor caudal ventrolateral medulla, were compared in spontaneously hypertensive rats (SHR) and in Wistar-Kyoto (WKY) rats. 2. 5-Fluoro indole-2-carboxylic acid elicited
R Bakshi et al.
Neuroscience letters, 110(1-2), 113-117 (1990-03-02)
Intrathecal (i.t.) administration of the opioid dynorphin causes neurological dysfunction and tissue damage. It has been suggested that these effects of dynorphin may be mediated, in part, by N-methyl-D-aspartate (NMDA) receptors. In the present studies, recently developed compounds that block
R Kamiński et al.
Journal of neural transmission (Vienna, Austria : 1996), 105(2-3), 133-146 (1998-07-11)
5-Fluoroindole-2-carboxylic acid, an antagonist of the glycine site within the NMDA receptor complex, administered intraperitoneally in doses of 150 and 200 mg/kg, 120 min before electroconvulsions, significantly raised the convulsive threshold from 6.8 to 7.9 and 8.3 mA, respectively. At
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service