All Photos(2)
About This Item
Empirical Formula (Hill Notation):
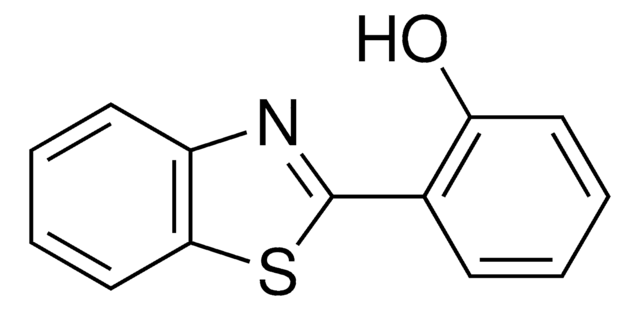
C13H9NS
CAS Number:
Molecular Weight:
211.28
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
111-113 °C (lit.)
functional group
phenyl
SMILES string
c1ccc(cc1)-c2nc3ccccc3s2
InChI
1S/C13H9NS/c1-2-6-10(7-3-1)13-14-11-8-4-5-9-12(11)15-13/h1-9H
InChI key
XBHOUXSGHYZCNH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Phenylbenzothiazole was used in the synthesis of two novel orange iridium(III) complexes, (bis[2-(biphenyl-3-yl)benzothiazole-N,C2′]iridium(III)(acetylacetonate) and bis[2-(4′-methoxybiphenyl-3-yl)benzothiazole-N,C2′]iridium(III) (acetylacetonate).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jun Dai et al.
Dalton transactions (Cambridge, England : 2003), 42(29), 10559-10571 (2013-06-14)
By introducing a phenyl substituent into the meta-site of the phenyl segment of the 2-phenylbenzothiazole ligand, two novel orange iridium(III) complexes, namely, (3Phbt)2Ir(acac) and (3OMePhbt)2Ir(acac), have been synthesized. Compared with their parent compound (bt)2Ir(acac), both of them possess much enhanced
Kuo-Shyan Lin et al.
Bioorganic & medicinal chemistry letters, 19(8), 2258-2262 (2009-03-17)
As a first step toward the development of (99m)Tc PiB analogs, we have synthesized six neutral Re 2-phenylbenzothiazoles via pendant or integrated approach. These Re compounds bind to Abeta(1-40) fibrils with fairly good affinities (K(i)=10.0-88.6nM) and have moderate lipophilicities (logP(C18)=1.21-3.26).
Rupam Sarma et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 77(1), 126-129 (2010-06-16)
The UV-visible and fluorescence properties of hydroxy substituted benzothiazole derivatives change on interactions with zinc(II), cadmium(II) and mercury(II) ions. The binding of 2-(2'-hydroxyphenyl)benzothiazole is specific to zinc(II) over cadmium(II) and mercury(II). Similar type of interactions of zinc(II), cadmium(II) and mercury(II)
Ming Li et al.
Dalton transactions (Cambridge, England : 2003), 40(27), 7153-7164 (2011-06-15)
Four novel iridium(III) complexes bearing biphenyl (7a-7c) or fluorenyl (7d) modified benzothiazole cyclometallate ligands are synthesized. In comparison with the yellow parent complex, bis(2-phenylbenzothiozolato-N,C(2')) iridium(III) (acetylacetonate) [(pbt)(2)Ir(acac)] (λ(PLmax) = 557 nm, φ(PL) = 0.26), 7a-7d show 20-43 nm bathochromic shifted
Rahmi Imamoglu et al.
Nature communications, 11(1), 365-365 (2020-01-19)
The ATP-dependent Hsp70 chaperones (DnaK in E. coli) mediate protein folding in cooperation with J proteins and nucleotide exchange factors (E. coli DnaJ and GrpE, respectively). The Hsp70 system prevents protein aggregation and increases folding yields. Whether it also enhances
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service