Recommended Products
biological source
goat
Quality Level
antibody form
purified immunoglobulin
clone
polyclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
ELISA: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
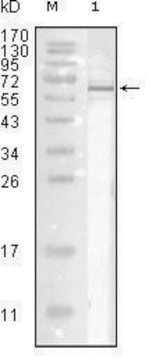
western blot: suitable
shipped in
wet ice
Specificity
Specific for Influenza A by IHA. Recognizes H1N1 and H3N2 and probably other Flu A strains. Non reactive with HEp-2 cells. May react with chicken cellular proteins. Does not react with Influenza B, RSV, Parainfluenza 1/2/3 or Adenovirus.
Immunogen
Influenza A-USSR (H1N1).
Application
Anti-Influenza A Antibody is an antibody against Influenza A for use in ELISA, IF, IH & WB.
Applications include ELISA, fluorescence microscopy, immunoblotting; unboiled samples in 1mM DTT and 2% SDS only and immunohistochemistry. Final working dilutions must be determined by end user.
Titer: 1:1,000 by indirect immunofluorescence. (1:2,500 by hemagglutination inhibition.)
Titer: 1:1,000 by indirect immunofluorescence. (1:2,500 by hemagglutination inhibition.)
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
Physical form
Format: Purified
Protein A Purified goat immunoglobulin in PBS (0.01 M, pH 7.2) with 0.1% sodium azide as a preservative.
Protein A purified
Storage and Stability
Maintain at +2–8°C for 3 months or at -20°C in aliquots for up to 12 months after date of receipt. Avoid repeated freeze/thaw cycles.
Analysis Note
Control
Influenza Control Slides, Catalogue Number 5010-5
Influenza Control Slides, Catalogue Number 5010-5
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
recommended
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiang Xu et al.
PloS one, 11(10), e0164501-e0164501 (2016-10-08)
Host-derived proteases can augment or help to clear infections. This dichotomy is exemplified by cathepsin L (CTSL), which helps Hendra virus and SARS coronavirus to invade cells, but is essential for survival in mice with mycoplasma pneumonia. The present study
Chih-Heng Huang et al.
The Journal of infectious diseases, 208(11), 1898-1905 (2013-08-01)
Reassortment within polymerase genes causes changes in the pathogenicity of influenza A viruses. We previously reported that the 2009 pH1N1 PA enhanced the pathogenicity of seasonal H1N1. We examined the effects of the PA gene from the HPAI H5N1 following
Mari Numata et al.
The Journal of biological chemistry, 295(6), 1704-1715 (2019-12-29)
The influenza A (H1N1)pdm09 outbreak in 2009 exemplified the problems accompanying the emergence of novel influenza A virus (IAV) strains and their unanticipated virulence in populations with no pre-existing immunity. Neuraminidase inhibitors (NAIs) are currently the drugs of choice for
H4N8 subtype avian influenza virus isolated from shorebirds contains a unique PB1 gene and causes severe respiratory disease in mice.
Bui, VN; Ogawa, H; Xininigen, ; Karibe, K; Matsuo, K; Awad, SS; Minoungou, GL; Yoden et al.
Virology null
Amy Y Chang et al.
Respiratory research, 17(1), 62-62 (2016-05-25)
The hexapeptide SLIGRL-amide activates protease-activated receptor-2 (PAR-2) and mas-related G protein-coupled receptor C11 (MRGPRC11), both of which are known to be expressed on populations of sensory nerves. SLIGRL-amide has recently been reported to inhibit influenza A (IAV) infection in mice
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service