The molarity of liquid Ethyl Methanesulfonate can be calculated using its density and molecular weight. The formula is: Molarity (moles/L) = Density (g/L) ÷ Molecular Weight (g/mol) × Purity. For this calculation: 1,206 g/L ÷ 124.16 g/mol × 0.994 = 9.65 M.
8.20774
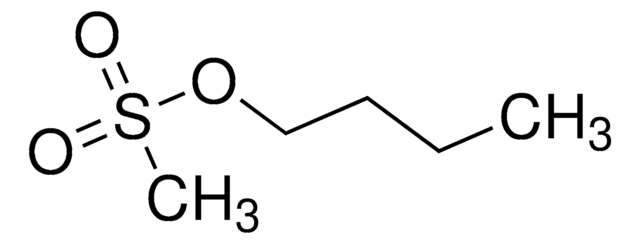
Ethyl methanesulfonate
for synthesis
Synonym(s):
Ethyl methanesulfonate, Methanesulfonic acid ethyl ester
About This Item
Recommended Products
vapor pressure
0.27 hPa ( 25 °C)
Quality Level
Assay
≥98.0% (GC)
form
liquid
bp
213-214 °C/1013 hPa
transition temp
flash point 100 °C
density
1.206 g/cm3 at 20 °C
storage temp.
2-30°C
SMILES string
[S](=O)(=O)(OCC)C
InChI
1S/C3H8O3S/c1-3-6-7(2,4)5/h3H2,1-2H3
InChI key
PLUBXMRUUVWRLT-UHFFFAOYSA-N
Analysis Note
Density (d 20 °C/ 4 °C): 1.205 - 1.207
Identity (IR): passes test
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Muta. 1B - Repr. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
212.0 °F
Flash Point(C)
100 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
-
What is the concentration or molarity of liquid EMS?
1 answer-
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service