All Photos(1)
About This Item
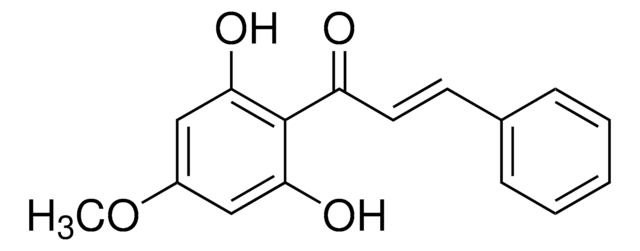
Linear Formula:
CH3OC6H4CH=CHCOC6H2(OCH3)2OH
CAS Number:
Molecular Weight:
314.33
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
112-116 °C (lit.)
functional group
ketone
SMILES string
COc1ccc(\C=C\C(=O)c2c(O)cc(OC)cc2OC)cc1
InChI
1S/C18H18O5/c1-21-13-7-4-12(5-8-13)6-9-15(19)18-16(20)10-14(22-2)11-17(18)23-3/h4-11,20H,1-3H3/b9-6+
InChI key
CGIBCVBDFUTMPT-RMKNXTFCSA-N
Related Categories
Application
2′-Hydroxy-4,4′,6′-trimethoxychalcone may be used to synthesize 2′,2”′-dihydroxy-4,4′,4′′,4”′,6′,6′′′-hexamethoxy[5′,5′′′]bichalcone and 3′-bromo-4,4′,6′-trimethoxy-2′-hydroxychalcone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Practical conversion of artemisinic acid in desoxyartemisinin.
Jung M, et al.
The Journal of Organic Chemistry, 51(26), 5417-5419 (1986)
Regioselective bromination of organic substrates by tetrabutylammonium bromide promoted by V2O5-H2O2: An environmentally favorable synthetic protocol.
Bora U, et al.
Organic Letters, 2(3), 247-249 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service