All Photos(1)
About This Item
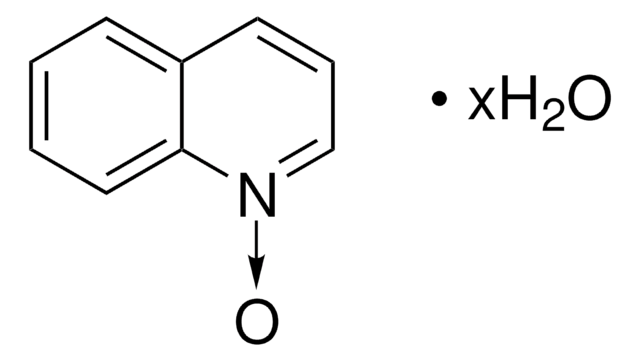
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105257
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
bp
270 °C (lit.)
mp
62-67 °C (lit.)
SMILES string
[O-][n+]1ccccc1
InChI
1S/C5H5NO/c7-6-4-2-1-3-5-6/h1-5H
InChI key
ILVXOBCQQYKLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyridine N-oxide axle with [2]rotaxanes was synthesized via an anion templated threading-followed-by-stoppering strategy.
Application
Pyridine N-oxide was used to study the FTIR spectra of pyridine N-oxide in acetonitrile.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
289.4 °F - closed cup
Flash Point(C)
143 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Masahito Murai et al.
Chemical communications (Cambridge, England), 48(61), 7622-7624 (2012-06-26)
Gold(I)-catalysed tandem oxygen-transfer/cycloisomerisation reaction of 2-(2-propynyl)pyridine N-oxides provides an atom-economical route to indolizinone frameworks.
Xue Gong et al.
Organic letters, 13(7), 1766-1769 (2011-03-11)
A Pd(II)-catalyzed oxidative coupling between pyridine N-oxides and N-substituted indoles via 2-fold C-H bond activation was achieved with high selectivity using Ag(2)CO(3) as an oxidant.
Jinshui Chen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(30), 7268-7276 (2009-07-07)
Optically active chiral alkyl chlorides are valuable compounds because of their bioactivity and versatile synthetic utility. Accordingly, the ring opening of epoxides with a chloride nucleophile stands as an important goal in asymmetric catalysis. We describe herein recent advances in
Santiago Barroso et al.
Organic letters, 13(3), 402-405 (2010-12-24)
Enantioselective nitrone cycloadditions with 2-alkenoyl pyridine N-oxides as dipolarophiles have been reported. The reaction is catalyzed by Cu(II)-BOX complexes to give the expected isoxazolidine products with high diastereo- and enantioselectivity.
Munawar Hussain et al.
Organic letters, 15(1), 54-57 (2012-12-22)
The synthesis of optically active piperidines by enantioselective addition of aryl Grignard reagents to pyridine N-oxides and lithium binolate followed by reduction is reported for the first time. The reaction results in high yields (51-94%) in combination with good ee
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service