M0285
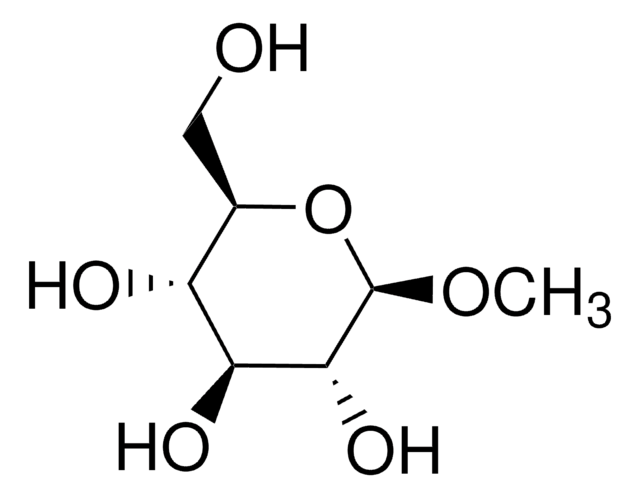
Methyl-β-D-galactopyranoside
Synonym(s):
Methyl β-D-galactoside
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14O6
CAS Number:
Molecular Weight:
194.18
Beilstein:
81569
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
Assay
≥98% (GC)
form
powder
technique(s)
gas chromatography (GC): suitable
color
white to faint yellow
mp
176-179 °C
solubility
water: 50 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3/t3-,4+,5+,6-,7-/m1/s1
InChI key
HOVAGTYPODGVJG-VOQCIKJUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
A β-D-galactopyranoside having a methyl substituent at the anomeric position.
Biochem/physiol Actions
Methyl galactoside is a hexose involved in the metabolism of 2-deoxygalactose.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T J Kelley et al.
Bioscience reports, 19(5), 433-447 (2000-04-14)
The highly purified DNA Pol-alpha from rat prostate tumor (PA-3) and human neuroblastoma (IMR-32) cells appeared to be inhibited by Ricin (RCA-II), and Con-A. Loss of activity (40 to 60%) of a specific form of DNA polymerase from IMR-32 was
N Declerck et al.
Protein engineering, 7(8), 997-1004 (1994-08-01)
The L-arabinose binding protein (ABP) of Escherichia coli naturally binds L-arabinose and D-galactose with very high affinity and, with reduced affinity, a variety of other sugars that differ only at the C5 position of the pyranose ring. However, there are
Alexander I Zinin et al.
Carbohydrate research, 337(7), 635-642 (2002-03-23)
1-O-Acetyl-beta-D-galactopyranose (AcGal), a new substrate for beta-galactosidase, was synthesized in a stereoselective manner by the trichloroacetimidate procedure. Kinetic parameters (K(M) and k(cat)) for the hydrolysis of 1-O-acetyl-beta-D-galactopyranose catalyzed by the beta-D-galactosidase from Penicillium sp. were compared with similar characteristics for
Yan Yang et al.
Carbohydrate research, 342(8), 1063-1070 (2007-03-16)
PIP60-1, a novel heteropolysaccharide isolated from fruiting bodies of the medicinal fungus, Phellinus igniarius, has a molecular weight of 1.71 x 10(4)Da and is composed of L-fucose, D-glucose, D-mannose, D-galactose and 3-O-Me-D-galactose in a ratio of 1:1:1:2:1. A structural investigation
Peter Capek
Carbohydrate research, 343(8), 1390-1393 (2008-04-25)
An arabinogalactan with a high content of 3-O-methyl-D-galactose residues has been isolated from the aerial parts of sage (Salvia officinalis L.). Structural studies of the polymer indicated a beta-1,6-D-galactopyranose backbone in which at least one-third of D-galactopyranosyl residues carries methoxyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service