L4919
Lysozyme from chicken egg white
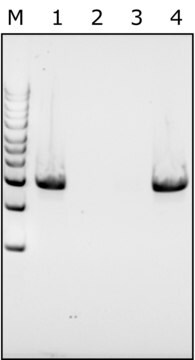
BioUltra, lyophilized powder, ≥98% (SDS-PAGE), ≥40,000 units/mg protein
Synonym(s):
Mucopeptide N-acetylmuramoylhydrolase, Muramidase
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
chicken egg white
Quality Level
grade
for molecular biology
product line
BioUltra
Assay
≥98% (SDS-PAGE)
form
lyophilized powder
specific activity
≥40,000 units/mg protein
mol wt
single-chain 14.3 kDa
composition
Protein, ≥90%
technique(s)
cell based assay: suitable
suitability
suitable for cell lysis
application(s)
cell analysis
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Lysozyme is abundantly found in animal and plant kingdoms. It is a natural food preservative and also found to be present in specific bacterial cell walls. Lysozyme is usually present in tears, milk, urine and saliva.
Application
Lysozyme from chicken egg white has been used:
- in dielectric spectroscopy studies of dynamics of protein
- as a control to measure the human lysozyme activity
- as a supplement in soaking solution to treat lenses
Enzyme breaks down the cell walls of bacteria; used to prepare spheroplasts.
Biochem/physiol Actions
Lysozyme hydrolyzes β(1→4) linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in peptidoglycan and between N-acetyl-D-glucosamine residues in chitodextrin. Gram-positive cells are quite susceptible to this hydrolysis as their cell walls have a high proportion of peptidoglycan. Gram-negative bacteria are less susceptible due to the presence of an outer membrane and a lower proportion of peptidoglycan. However, these cells may be hydrolyzed in the presence of EDTA that chelates metal ions in the outer bacterial membrane.
The enzyme is active over a broad pH range (6.0 to 9.0). At pH 6.2, maximal activity is observed over a wider range of ionic strengths (0.02 to 0.100 M) than at pH 9.2 (0.01 to 0.06 M).
The enzyme is active over a broad pH range (6.0 to 9.0). At pH 6.2, maximal activity is observed over a wider range of ionic strengths (0.02 to 0.100 M) than at pH 9.2 (0.01 to 0.06 M).
Lysozymes participate in the defense mechanism. It has the ability to stimulate catalysis by bringing steric stress in the substrates.
Features and Benefits
- Highly purified by repeated crystallization and dialysis
- Each lot is use-tested for isolation of plasmid DNA from E. coli
Unit Definition
One unit will lyse 0.6 μg of Micrococcus lysodeikticus per minute by turbidimetric detection at 600 nm when suspended in buffer at pH 6.2 at 25 °C.
Preparation Note
3× crystallized
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Corneal cell adhesion to contact lens hydrogel materials enhanced via tear film protein deposition
Elkins CM, et al.
Testing, e105512-e105512 (2014)
Metabolic engineering of probiotic Saccharomyces boulardii
Liu JJ, et al.
Applied and Environmental Microbiology, 82(8), 2280-2287 (2016)
Natural Food Antimicrobial Systems (2000)
Jordi van Gestel et al.
Nature ecology & evolution, 3(8), 1184-1196 (2019-07-25)
Microbes are exposed to changing environments, to which they can respond by adopting various lifestyles such as swimming, colony formation or dormancy. These lifestyles are often studied in isolation, thereby giving a fragmented view of the life cycle as a
Protein dynamics in a broad frequency range: Dielectric spectroscopy studies
Nakanishi M and Sokolov AP
Journal of Non-Crystalline Solids, 407, 478-485 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service