I100
5-Iodotubercidin
≥85%, solid
Synonym(s):
4-Amino-5-iodo-7-(β-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine, 5-Iodotubericidin, 7-Iodo-7-deazaadenosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H13IN4O4
CAS Number:
Molecular Weight:
392.15
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
form:
solid
Assay:
≥85%
Recommended Products
Quality Level
Assay
≥85%
form
solid
storage temp.
2-8°C
SMILES string
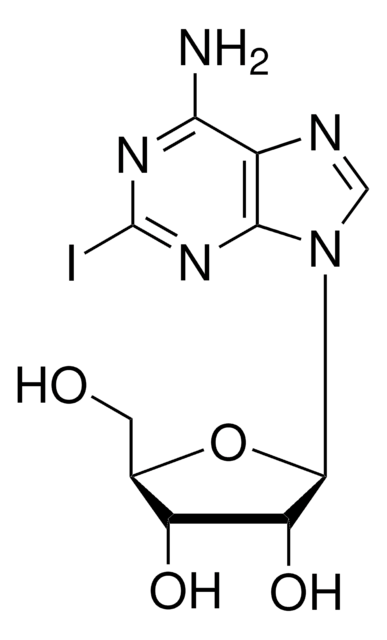
Nc1ncnc2n(cc(I)c12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7-,8-,11-/m1/s1
InChI key
WHSIXKUPQCKWBY-IOSLPCCCSA-N
General description
5-Iodotubercidin is a purine and modulates cellular adenosine levels by inhibiting the functionality of adenosine kinase. It is an activator for p53 and favors tumor suppression and may serve as a potential chemotherapeutic agent. 5-Iodotubercidin mediated blockade of adenosine to adenosine monophosphate (AMP) conversion results in alleviating antinociceptive effect of AMP. 5-Iodotubercidin is under clinical trial testing for treating epilepsy.
solubility: 10 mg/mL in DMSO
Application
5-Iodotubercidin has been used for the inhibition of retinoblastoma cells, astroglial cultures and for the inhibition of adenosine kinase in human umbilical vein endothelial cells (HUVECs).
Biochem/physiol Actions
Potent inhibitor of adenosine uptake into brain, and of adenosine kinase and subsequent metabolism of adenine nucleotides. In cultured rat hepatocytes, 5-iodotubercidin inhibits both acetyl-CoA carboxylase and de novo synthesis of fatty acids and cholesterol.
5-Iodotubercidin increases fatty acid oxidation activity and glycogen synthesis in hepatocytes. 5-Iodotubercidin is also a potent inhibitor of adenosine uptake into brain.
Caution
Solutions may be stored frozen. Use promptly when thawed and protect from exposure to light.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Understanding the basic mechanisms underlying seizures in mesial temporal lobe epilepsy and possible therapeutic targets: a review
O'dell CM, et al.
Journal of Neuroscience Research, 90(5), 913-924 (2012)
Ecto-5?-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits
Sowa NA, et al.
The Journal of Neuroscience, 30(6), 2235-2244 (2010)
Opposite modulation of astroglial proliferation by adenosine 5?-O-(2-thio)-diphosphate and 2-methylthioadenosine-5?-diphosphate: mechanisms involved
Quintas C, et al.
Neuroscience, 182(8), 32-42 (2011)
D Massillon et al.
The Biochemical journal, 299 ( Pt 1), 123-128 (1994-04-01)
Addition of micromolar concentrations of the adenosine derivative 5-iodotubercidin (Itu) initiates glycogen synthesis in isolated hepatocytes by causing inactivation of phosphorylase and activation of glycogen synthase [Flückiger-Isler and Walter (1993) Biochem. J. 292, 85-91]. We report here that Itu also
Retinoblastoma cells are inhibited by aminoimidazole carboxamide ribonucleotide (AICAR) partially through activation of AMP-dependent kinase
Theodoropoulou S, et al.
Faseb Journal, 24(8), 2620-2630 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service