G2785
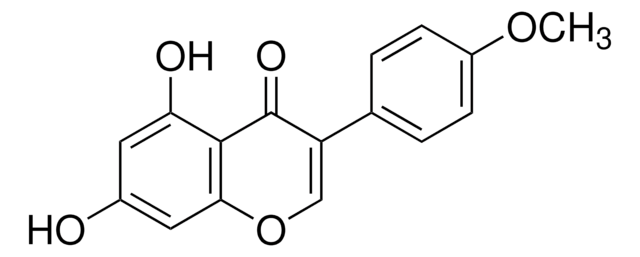
Glycitein
≥97% (HPLC)
Synonym(s):
4′,7-Dihydroxy-6-methoxyisoflavone, Glycetein
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H12O5
CAS Number:
Molecular Weight:
284.26
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥97% (HPLC)
form
powder
solubility
DMF: 1 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
COc1cc2C(=O)C(=COc2cc1O)c3ccc(O)cc3
InChI
1S/C16H12O5/c1-20-15-6-11-14(7-13(15)18)21-8-12(16(11)19)9-2-4-10(17)5-3-9/h2-8,17-18H,1H3
InChI key
DXYUAIFZCFRPTH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Glycitein has been used as standard for the analysis of soy isoflavones and metabolites in urine. It has also been used to bind to recombinant estrogen and progesterone receptors to know the relative binding affinity (RBA) for detection of potential endocrine disruptors.
Biochem/physiol Actions
Glycitein is a soybean (yellow cultivar) isoflavonoid; its natural glycosides are synergistic with genistein in inducing specific gene expression. Glycitein may be used to study anti-oxidation processes at the level of gene transcription where it increases the binding of transcription factors [nuclear factor-E2-related factor 2 (Nrf2) and c-Jun] to the antioxidant response element (ARE) on HO-1 and NQO1 promoters. Glycitein may be used in combination with other isoflavonoids such as genistein and daidzein to study apoptosis.
Glycitein possesses weak estrogenic activity than other soy isoflavones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Smit et al.
The Journal of biological chemistry, 267(1), 310-318 (1992-01-05)
Besides genistein and daidzein, which are active inducers of the nodYABCSUIJ operon in Bradyrhizobium japonicum, soybean seeds also excrete compounds that are not inducers of the nodYABCSUIJ genes but enhance induction of this operon in the presence of a suboptimal
Andrean L Simons et al.
Journal of agricultural and food chemistry, 53(22), 8519-8525 (2005-10-27)
Gut microbial disappearance and metabolism of the soy isoflavone glycitein, 7,4'-dihydroxy-6-methoxyisoflavone, were investigated by incubating glycitein anaerobically with feces from 12 human subjects. The subjects' ages ranged from 24 to 53 years with a body mass index (BMI) of 20.9-25.8
Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors
Scippo M L, et al.
Analytical and Bioanalytical Chemistry, 378(3), 664-669 (2004)
Jin-Sun Park et al.
Journal of neurochemistry, 119(5), 909-919 (2011-07-26)
The brain is highly vulnerable to oxidative stress, thus controlling oxidative stress is considered to be an important therapeutic target for neurodegenerative diseases. In this study, we found that two isoflavone metabolites (tectorigenin and glycitein) inhibited hydrogen peroxide-induced reactive oxygen
Soy isoflavone metabolism in cats compared with other species: urinary metabolite concentrations and glucuronidation by liver microsomes
Redmon J M, et al.
Xenobiotica, 46(5), 406-415 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service