C1999
CMP-Sialic Acid Synthetase from Neisseria meningitidis group B
recombinant, expressed in E. coli BL21, ≥10 units/mg protein
Synonym(s):
CTP: N-Acylneuraminate cytidylyltransferase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli BL21
Quality Level
form
lyophilized solid
specific activity
≥10 units/mg protein
mol wt
26.0 kDa
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Cytidine monophosphate (CMP)-Sialic Acid Synthetase from Neisseria meningitidis group B is encoded in neuA gene. The protein has a molecular weight of 24.8 kDa.
Application
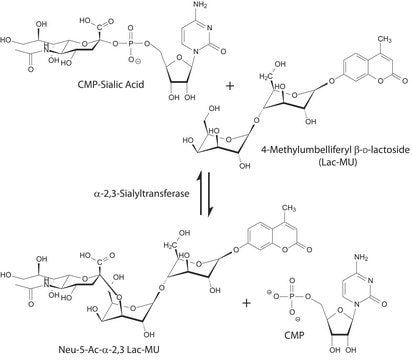
The enzyme has been utilized to synthesize CMP-sialic acid and its derivatives.
Biochem/physiol Actions
Cytidine monophosphate (CMP)-sialic acid synthetase catalyses the conversion of N?acetylneuraminic acid (NeuNAc) to CMP-NeuNAc. CMP-sialic acid synthetase has globular α/β domain and is categorised under αβα three-layered sandwich fold. The dimerization domain aids the interaction between the monomers. It also has mononucleotide binding and NeuAc binding pocket. Mg2+ is essential for the catalytic functionality of CMP-sialic acid synthetase.
Unit Definition
One unit will catalyze the formation of 1 μmol CMP-Neu-5-Ac from Neu-5-Ac and CTP per minute at 37 °C at pH 8.0.
Physical form
Supplied as a lyophilized powder containing Tris-HCl and NaCl.
Analysis Note
Enzymatic activity assays are performed in Tris-HCl buffer (100 mM, pH 8.5) containing Neu-5-Ac (1 mM) and CTP (1 mM) at 37 °C for 30 min and analyzed using capillary electrophoresis with a UV detector (200 nm).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jessica H Wong et al.
Organic & biomolecular chemistry, 7(1), 27-29 (2008-12-17)
A modular replacement approach to the synthesis of sulfo-nucleotide analogs prepared from condensation of nucleoside aldehydes with bis phosphonate Horner-Wadsworth-Emmons reagents is disclosed. These analogs were shown to be inhibitors of Neisseria meningitidis CSS (NmCSS), which is a key enzyme
Hai Yu et al.
Bioorganic & medicinal chemistry, 12(24), 6427-6435 (2004-11-24)
Three C terminal His6-tagged recombinant microbial CMP-sialic acid synthetases [EC 2.7.7.43] cloned from Neisseria meningitidis group B, Streptococcus agalactiae serotype V, and Escherichia coli K1, respectively, were evaluated for their ability in the synthesis of CMP-sialic acid derivatives in a
Purification and characterization of the recombinant CMP-sialic acid synthetase from Neisseria meningitides.
Gilbert, M., et al
Biotechnology Letters, 19, 417-420 (1997)
CMP-sialic acid synthetase: the point of constriction in the sialylation pathway
SialoGlyco Chemistry and Biology I, 139-167 (2013)
Sandra Ratzow et al.
Journal of clinical microbiology, 45(6), 1965-1968 (2007-04-06)
The standard sequence-based method for the typing of Legionella pneumophila serogroup 1 strains was extended by using the gspA and neuA alleles. The use of neuA as a seventh allele for typing significantly increased the index of discrimination calculated for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service