I5109
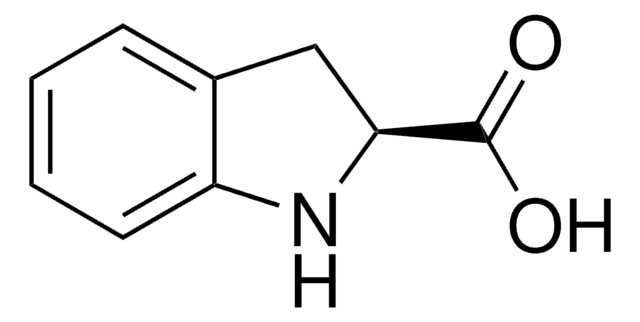
Indole-2-carboxylic acid
98%
Synonym(s):
2-Carboxyindole, 2-Indolylformic acid, NSC 16598
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C9H7NO2
CAS Number:
Molecular Weight:
161.16
Beilstein:
124132
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
202-206 °C (lit.)
SMILES string
OC(=O)c1cc2ccccc2[nH]1
InChI
1S/C9H7NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-5,10H,(H,11,12)
InChI key
HCUARRIEZVDMPT-UHFFFAOYSA-N
Gene Information
human ... SRD5A1(6715)
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Reactant for total synthesis of (±)-dibromophakellin and analogs
- Reactant for synthesis of the pyrrolizidine alkaloid (±)-trachelanthamidine
- Reactant for stereoselective preparation of renieramycin G analogs
- Reactant for preparation of spirooxoindolepyrrolidines via reduction of indole-2-carboxylic acid followed by oxidation, condensation, reduction, amidation and Kharasch radical cyclization
- Reactant for Pd-catalyzed cyclization
- Reactant for preparation of N,N′-(pentane)diylbis[indolecarboxamide] and N,N′-[phenylenebis(methylene)]bis[indolecarboxamide] derivatives
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Di Fabio et al.
Journal of medicinal chemistry, 40(6), 841-850 (1997-03-14)
A series of indole-2-carboxylates bearing suitable chains at the C-3 position of the indole nucleus was synthesized and evaluated in terms of in vitro affinity using [3H]glycine binding assay and in vivo potency by inhibition of convulsions induced by N-methyl-D-aspartate
Stephen P Nighswander-Rempel et al.
Photochemistry and photobiology, 84(3), 613-619 (2008-01-23)
We have synthesized a compound ideally suited to the study of structure-function relationships in eumelanin synthesis. N-methyl-5-hydroxy-6-methoxy-indole (MHMI) has key functional groups strategically placed on the indole framework to hinder binding in the 2, 5, 6 and 7 positions. Thus
C Kuehm-Caubere et al.
Journal of medicinal chemistry, 40(8), 1201-1210 (1997-04-11)
Series of indole-2-carboxamide and cycloalkeno[1,2-b]indole derivatives were synthesized and evaluated in order to determine the necessary structural requirements for a high inhibition of human LDL copper-induced peroxidation. Various modulations were systematically performed on the indole and cycloalkeno[1,2-b]indole nuclei as well
R Rama Suresh et al.
The Journal of organic chemistry, 77(16), 6959-6969 (2012-07-26)
Two methodologies, one involving Ar-I reactivity and the other through C-H functionalization, for the formation of indolo[2,3-c]pyrane-1-ones via the corresponding allenes, are presented. A highly efficient approach to indolo[2,3-c]pyrane-1-one derivatives through the Pd-catalyzed regioselective annulation of allenes with 3-iodo-1-alkylindole-2-carboxylic acids
Gopinadhan N Anilkumar et al.
Bioorganic & medicinal chemistry letters, 21(18), 5336-5341 (2011-08-16)
SAR development of indole-based palm site inhibitors of HCV NS5B polymerase exemplified by initial indole lead 1 (NS5B IC(50)=0.9 μM, replicon EC(50)>100 μM) is described. Structure-based drug design led to the incorporation of novel heterocyclic moieties at the indole C3-position
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service