D97754
Diethyl malonate
ReagentPlus®, 99%
Synonym(s):
Malonic acid diethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
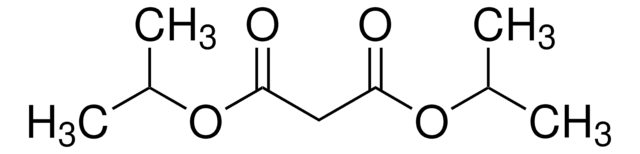
CH2(COOC2H5)2
CAS Number:
Molecular Weight:
160.17
Beilstein:
774687
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
Assay:
99%
Recommended Products
vapor density
5.52 (vs air)
Quality Level
vapor pressure
1 mmHg ( 40 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
refractive index
n20/D 1.413 (lit.)
bp
199 °C (lit.)
mp
−51-−50 °C (lit.)
density
1.055 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)CC(=O)OCC
InChI
1S/C7H12O4/c1-3-10-6(8)5-7(9)11-4-2/h3-5H2,1-2H3
InChI key
IYXGSMUGOJNHAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Diethyl malonate is diethyl ester of malonic acid. It is widely used as a versatile building block for introducing the malonate functional group into molecules. Acylation of diethyl malonate using magnesium chloride and triethylamine is reported. K2CO3-catalyzed 1,4-addition reaction of diethyl malonate with various substituted 1,2-allenic ketones yields polyfunctionalized β,γ-unsaturated enones.
Application
Diethyl malonate was used to investigate Knoevenagel condensation reactions in microreactor using zeolite catalysts obtained by grafting amino groups onto NaX and CsNaX zeolites. It may be used in the synthesis of diethyl benzylidenemalonate by base catalyzed Knoevenagel condensation with benzaldehyde. It may be used in the synthesis of α-aryl malonates.
Packaging
Packaged in glass bottles
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shengming Ma et al.
The Journal of organic chemistry, 68(23), 8996-9002 (2003-11-08)
The K2CO3 (10 mol %)-catalyzed 1,4-addition reaction of diethyl malonate with various substituted 1,2-allenic ketones leading to polyfunctionalized beta,gamma-unsaturated enones 3 or 4 was studied. With 3-unsubstituted 1-substituted-1,2-allenyl ketones, the highly selective formation of beta,gamma-unsaturated enones 4 was observed; with
Solvent-free organocatalytic Michael addition of diethyl malonate to nitroalkenes: the practical synthesis of Pregabalin and ?-nitrobutyric acid derivatives
J Liu, et al.
Tetrahedron, 67, 636-640 (2011)
Edward J Hennessy et al.
Organic letters, 4(2), 269-272 (2002-02-14)
[reaction: see text] A general method for the synthesis of alpha-aryl malonates is described. The coupling of an aryl iodide and diethyl malonate in the presence of Cs(2)CO(3) and catalytic amounts of copper(I) iodide and 2-phenylphenol affords the alpha-aryl malonate
Integration of heterogeneous catalysts into complex synthetic routes: sequential vs. one-pot reactions in a (Knoevenagel+ Mukaiyama-Michael+ hydrogenation+ transesterification) sequence.
Fraile JM, et al.
Catalysis Science & Technology, 3(2), 436-443 (2013)
Lian Jin Liu et al.
Nucleosides, nucleotides & nucleic acids, 30(10), 784-797 (2011-10-05)
Novel 5'-norcarbocyclic adenosine phosphonic acid analogues with 6'-electropositive moiety such as spirocyclopropane were designed and synthesized from the commercially available diethylmalonate 5. Regioselective Mitsunobu reaction proceeded in the presence of an allylic functional group at a low reaction temperature in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service