696307
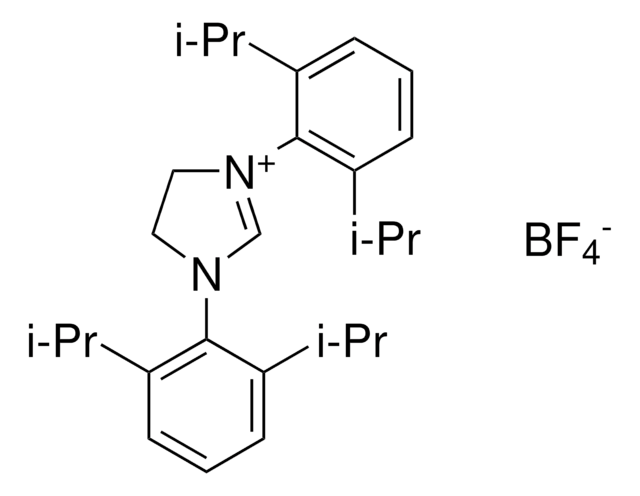
Chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I)
Synonym(s):
[(iPr)CuCl], [1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I) chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C27H36ClCuN2
CAS Number:
Molecular Weight:
487.59
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
reaction suitability
core: copper
reagent type: catalyst
mp
>300 °C
SMILES string
CC(C)c1cccc(C(C)C)c1N2C=CN(\C2=[Cu]\Cl)c3c(cccc3C(C)C)C(C)C
InChI
1S/C27H36N2.ClH.Cu/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;;/h9-16,18-21H,1-8H3;1H;/q;;+1/p-1
InChI key
JPUFNIIPFXQOCB-UHFFFAOYSA-M
Application
"Use for reduction of olefin and carbonyl, carbene transfer reaction, aziridination of olefins, and methyleneation of aldehydes."
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kaur H. et al.
Organometallics, 23, 1157-1157 (2004)
Manuel R Fructos et al.
Journal of the American Chemical Society, 126(35), 10846-10847 (2004-09-02)
The complex IPrCuCl (1) catalyzes the transfer of the :CHCO2Et group from ethyl diazoacetate (EDA) to unsaturated and saturated substrates (olefins, amine, alcohols) with very high yields. In the absence of substrate, the complex 1 does not react with EDA
Hélène Lebel et al.
The Journal of organic chemistry, 72(1), 144-149 (2006-12-30)
(NHC)-Cu (NHC = N-heterocyclic carbene) complexes efficiently catalyzed the methylenation of a variety of aliphatic and aromatic aldehydes and ketones in the presence of trimethylsilyldiazomethane, triphenylphosphine, and 2-propanol. The copper catalysts are not only inexpensive compared to rhodium complexes, but
Barry M Trost et al.
Journal of the American Chemical Society, 128(18), 6054-6055 (2006-05-04)
New classes of nucleophiles, pyrroles, and N-methoxyamides were developed for Pd-catalyzed AAA reactions. By varying the functional groups at the 2-position of pyrroles, either regioisomer of the piperazinone is available. Using one regioisomer, the total synthesis of (+)-agelastatin A in
Valdas Jurkauskas et al.
Organic letters, 5(14), 2417-2420 (2003-07-05)
[reaction: see text] An N-heterocyclic carbene copper chloride (NHC-CuCl) complex (2) has been prepared and used to catalyze the conjugate reduction of alpha,beta-unsaturated carbonyl compounds. The combination of catalytic amounts of 2 and NaOt-Bu with poly(methylhydrosiloxane) (PMHS) as the stoichiometric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)
![Chloro[1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]copper(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/160/888/97509eeb-0719-4853-aaae-8a9d02f4f7ad/640/97509eeb-0719-4853-aaae-8a9d02f4f7ad.png)

![[1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene] [bis(trifluoromethanesulfonyl)imide]gold(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/336/250/c96c15b3-1a1c-479f-a588-76e98905be23/640/c96c15b3-1a1c-479f-a588-76e98905be23.png)



![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)