662623
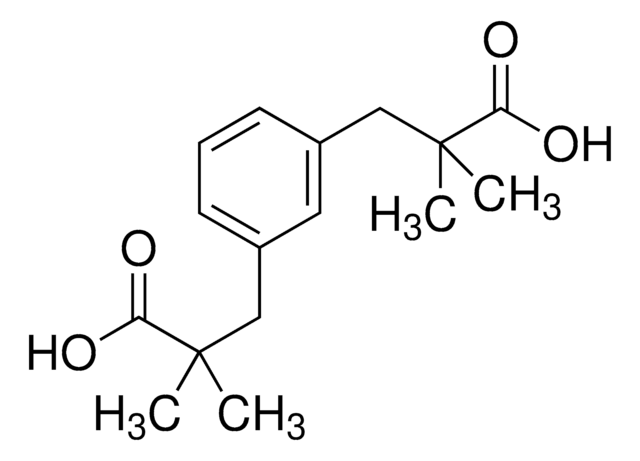
Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)]
95%
Synonym(s):
Rh2(esp)2
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C32H40O8Rh2
CAS Number:
Molecular Weight:
758.47
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
reaction suitability
core: rhodium
reagent type: catalyst
reaction type: C-H Activation
mp
>300 °C
SMILES string
[Rh][Rh].CC(C)(Cc1cccc(CC(C)(C)C(O)=O)c1)C(O)=O.CC(C)(Cc2cccc(CC(C)(C)C(O)=O)c2)C(O)=O
InChI
1S/2C16H22O4.2Rh/c2*1-15(2,13(17)18)9-11-6-5-7-12(8-11)10-16(3,4)14(19)20;;/h2*5-8H,9-10H2,1-4H3,(H,17,18)(H,19,20);;
InChI key
OBMUTUNJWNQIAJ-UHFFFAOYSA-N
General description
Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionicacid)] has been identified as efficient C-H activation catalyst.
Application
Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionicacid)] is used as a catalyst for oxidative C−H amination of urea and guanidinederived substrates to yield corresponding heterocyclic products.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kristin Williams Fiori et al.
Journal of the American Chemical Society, 129(3), 562-568 (2007-01-18)
Reaction methodology for intermolecular C-H amination of benzylic and 3 degrees C-H bonds is described. This process uses the starting alkane as the limiting reagent, gives optically pure tetrasubstituted amines through stereospecific insertion into enantiomeric 3 degrees centers, displays high
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service