529249
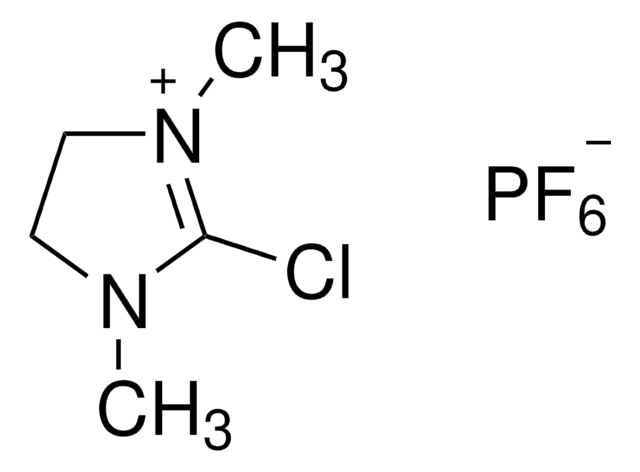
2-Chloro-1,3-dimethylimidazolinium chloride
for peptide synthesis
Synonym(s):
2-Chloro-4,5-dihydro-1,3-dimethyl-1H-imidazolium chloride, DMC
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H10Cl2N2
CAS Number:
Molecular Weight:
169.05
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
2-Chloro-1,3-dimethylimidazolinium chloride,
form
crystalline
Quality Level
reaction suitability
reaction type: Coupling Reactions
mp
133-140 °C (lit.)
application(s)
peptide synthesis
functional group
chloro
SMILES string
[Cl-].CN1CC[N+](C)=C1Cl
InChI
1S/C5H10ClN2.ClH/c1-7-3-4-8(2)5(7)6;/h3-4H2,1-2H3;1H/q+1;/p-1
InChI key
AEBBXVHGVADBHA-UHFFFAOYSA-M
Application
Reagent for synthesis of:
Tagged glucose as an intermediate in the synthesis of branched oligosaccharides
Fluorescent chemosensors
1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1
Allosteric glucokinase activators
Reactant for synthesis of:
Organic azides from primary amines
Reagent for aza-Henry reactions
Tagged glucose as an intermediate in the synthesis of branched oligosaccharides
Fluorescent chemosensors
1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1
Allosteric glucokinase activators
Reactant for synthesis of:
Organic azides from primary amines
Reagent for aza-Henry reactions
Activating agent in total synthesis of macroviracin A, cycloviracin B1, and cyclic silanes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alois Fürstner et al.
Journal of the American Chemical Society, 125(43), 13132-13142 (2003-10-23)
The first total synthesis of the antivirally active glycolipid cycloviracin B(1) (1) is described. The approach is based on a two-directional synthesis strategy which constructs the C(2)()-symmetrical macrodiolide core of the target by an efficient template-directed macrodilactonization reaction promoted by
Journal of Organometallic Chemistry, 686, 175-182 (2003)
Shunya Takahashi et al.
The Journal of organic chemistry, 69(13), 4509-4515 (2004-06-19)
The C(2)-symmetric macrodiolide core 2 of an antiviral agent, macroviracin A (1), was constructed in a single step by the intermolecular macrodimerization of C(22)-hydroxy carboxylic acid 3 with 2-chloro-1,3-dimethylimidazolinium chloride and DMAP in the presence of sodium hydride (NaH). The
Yanzi Gou et al.
Journal of polymer science. Part A, Polymer chemistry, 51(12), 2588-2597 (2013-06-14)
Synthetic glycopolymers are important natural oligosaccharides mimics for many biological applications. To develop glycopolymeric drugs and therapeutic agents, factors that control the receptor-ligand interaction need to be investigated. A library of well-defined glycopolymers has been prepared by the combination of
Shonoi A Ming et al.
Glycobiology, 28(2), 100-107 (2017-12-12)
Neisseria meningitidis Group X is an emerging cause of bacterial meningitis in Sub-Saharan Africa. The capsular polysaccharide of Group X is a homopolymer of N-acetylglucosamine α(1-4) phosphate and is a vaccine target for prevention of disease associated with this meningococcal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service