All Photos(2)
About This Item
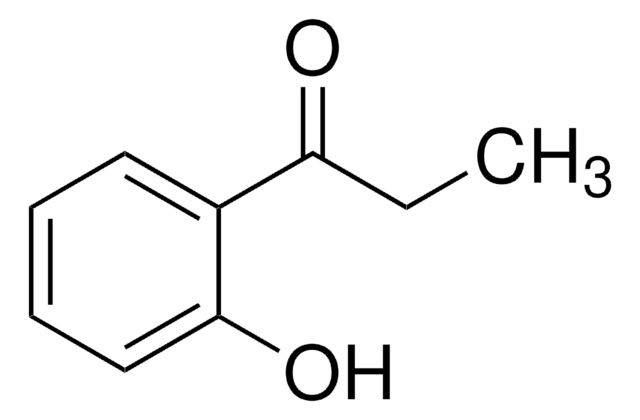
Linear Formula:
C6H5COCH2OH
CAS Number:
Molecular Weight:
136.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
86-89 °C (lit.)
functional group
hydroxyl
ketone
phenyl
SMILES string
OCC(=O)c1ccccc1
InChI
1S/C8H8O2/c9-6-8(10)7-4-2-1-3-5-7/h1-5,9H,6H2
InChI key
ZWVHTXAYIKBMEE-UHFFFAOYSA-N
Application
2-Hydroxyacetophenone can be used as a starting material for the synthesis of:
- Enantioselective 1R-phenyl-1,2-ethanediol in the presence of a rhodium(III) catalyst by asymmetric transfer hydrogenation.
- Copper(II) complexes of 2-hydroxyacetophenone N-substituted thiosemicarbazones.
- Chromium, molybdenum, and ruthenium complexes of 2-hydroxyacetophenone Schiff bases.
- 2-Hydroxyacetophenone-aroyl hydrazone derivatives for inhibition of copper corrosion in nitric acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic studies on optically active Schiff-base ligands derived from condensation of 2-hydroxyacetophenone and chiral diamines.
Gao WT and Zheng Z.
Molecules (Basel), 7(7), 511-516 (2002)
2-Hydroxyacetophenone-aroyl hydrazone derivatives as corrosion inhibitors for copper dissolution in nitric acid solution
AS Fouda, et al.
Bulletin of the Korean Chemical Society,, 21(11), 1085-1089 (2000)
Mateusz Łużny et al.
Molecules (Basel, Switzerland), 24(17) (2019-09-05)
Biotransformations were performed on eight selected yeast strains, all of which were able to selectively hydrogenate the chalcone derivatives 3-(2"-furyl)- (1) and 3-(2"-thienyl)-1-(2'-hydroxyphenyl)-prop-2-en-1-one (3) into 3-(2"-furyl)- (2) and 3-(2"-thienyl)-1-(2'-hydroxyphenyl)-propan-1-one (4) respectively. The highest efficiency of hydrogenation of the double bond
A stereochemically well-defined rhodium (III) catalyst for asymmetric transfer hydrogenation of ketones
Matharu DS, et al.
Organic Letters, 7(24), 5489-5491 (2005)
Synthesis and structural characterization of mixed ligand ? 1-2-hydroxyacetophenone complexes of cobalt (III).
Mondal N, et al.
Polyhedron, 19(28), 2707-2711 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service