418218
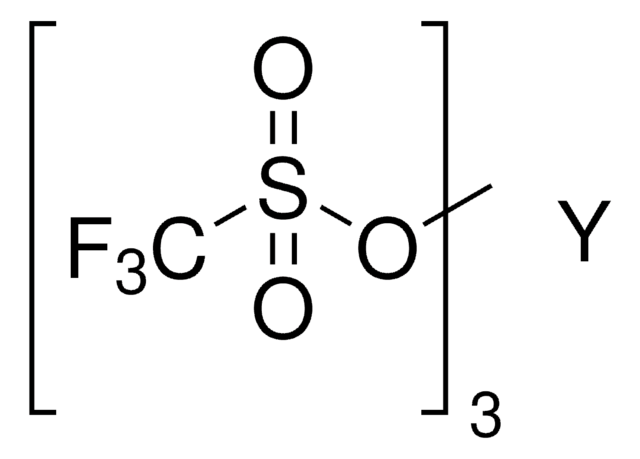
Scandium(III) triflate
99%
Synonym(s):
Sc(OTf)3, Scandium(III) trifluoromethanesulfonate, Trifluoromethanesulfonic acid scandium(III) salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Sc(SO3CF3)3
CAS Number:
Molecular Weight:
492.16
Beilstein:
8510151
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
reaction suitability
core: scandium
reagent type: catalyst
SMILES string
[Sc+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
HZXJVDYQRYYYOR-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates.
Application
Scandium(III) triflate was used as a catalyst in:
- Hydrothiolation reaction of aromatic and aliphatic thiols.
- Selective two-electron reduction of O2 by ferrocene derivatives.
- Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
- Synthesis of β-cyanoketones.
- Combination with triethylsilane to reductively open functionalized pyranoside rings.
- The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Giovanni Desimoni et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(12), 3630-3636 (2008-02-29)
The asymmetric Friedel-Crafts reaction between a series of substituted indoles 2 a-l and methyl (E)-2-oxo-4-aryl-3-butenoates 3 a-c has been efficiently catalyzed by the scandium(III) triflate complex of (4'S,5'S)-2,6-bis[4'-(triisopropylsilyl)oxymethyl-5'-phenyl-1',3'-oxazolin-2'-yl]pyridine (pybox; 1). Substituted 4-(indol-3-yl)-2-oxo-4-arylbutyric acid methyl esters 4 a-n were usually formed
Mild reductive opening of aryl pyranosides promoted by scandium(III) triflate.
Hua-Li Qin et al.
Journal of the American Chemical Society, 129(1), 38-39 (2007-01-04)
Jens Oelerich et al.
Organic & biomolecular chemistry, 13(9), 2793-2799 (2015-01-22)
Alkylidene malonates and α,β-unsaturated α'-hydroxyketones are demonstrated to be efficient classes of electrophiles for the scandium(III) triflate/sodium dodecyl sulphate (SDS) catalysed vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water. These substrates contain an easily removable auxiliary group that increases
Saya Kakuda et al.
Journal of the American Chemical Society, 137(9), 3330-3337 (2015-02-11)
Mononuclear copper complexes, [(tmpa)Cu(II)(CH3CN)](ClO4)2 (1, tmpa = tris(2-pyridylmethyl)amine) and [(BzQ)Cu(II)(H2O)2](ClO4)2 (2, BzQ = bis(2-quinolinylmethyl)benzylamine)], act as efficient catalysts for the selective two-electron reduction of O2 by ferrocene derivatives in the presence of scandium triflate (Sc(OTf)3) in acetone, whereas 1 catalyzes
Alex R Lippert et al.
Journal of the American Chemical Society, 128(46), 14738-14739 (2006-11-16)
Oligosubstituted bullvalones were synthesized in eight steps from 2,6-cycloheptadienone via a unique Lewis acid catalyzed intramolecular cyclopropanation of a stabilized sulfur ylide, leading directly to the tetracyclic cage structure. Upon exposure to base, the substituted bullvalones tautomerized to a hydroxybullvalene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service