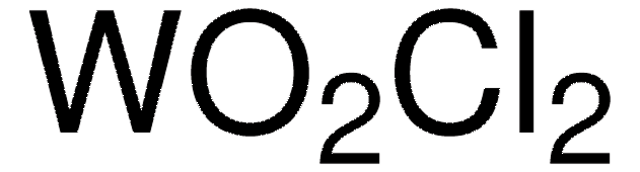373729
Molybdenum(VI) tetrachloride oxide
97%
Synonym(s):
Molybdenum chloride oxide, Molybdenum oxide chloride, Molybdenum oxytetrachloride, Molybdenum tetrachloride monoxide, Molybdenum(VI) oxychloride, Tetrachlorooxomolybdenum
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
MoOCl4
CAS Number:
Molecular Weight:
253.75
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder or crystals
reaction suitability
reagent type: catalyst
core: molybdenum
storage temp.
2-8°C
SMILES string
Cl[Mo](Cl)(Cl)(Cl)=O
InChI
1S/4ClH.Mo.O/h4*1H;;/q;;;;+4;/p-4
InChI key
UYEGPKGLVUUIGD-UHFFFAOYSA-J
Application
Molybdenum(VI) tetrachloride oxide can be used:
- To prepare a binary metal catalyst (MoOCl4-n-BuLi) for the living polymerization of various acetylenes.
- As an oxidant in the aromatization of various Hantzsch-1,4-dihydropyridines.
- As a molybdenum (Mo) precursor to prepare nafion-Mo composite material, which is used as a catalyst for the preparation of nitrones by reacting primary amines with aldehydes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nafion supported molybdenum oxychloride: Recyclable catalyst for one-pot synthesis of nitrones via direct condensation/oxidation of primary amines and aldehydes using UHP as oxidant
Singh B, et al.
Green Chemistry, 11(12), 1941-1944 (2009)
Living Polymerization of Substituted Acetylenes by a Novel Binary Catalyst, MoOCl4- n-BuLi
Hayano S and Masuda T
Macromolecules, 31(10), 3170-3174 (1998)
Hayano, S. Masuda, T.
Macromolecules, 31, 3170-3170 (1998)
Mladen Litvić et al.
Bioorganic & medicinal chemistry letters, 22(11), 3676-3681 (2012-05-02)
Mo(VI) and Mo(V) salts both react selectively with Hantzsch esters to produce substitute pyridines in good-to-excellent yield (75-99%). The remarkable reactivity and selectivity of MoOCl(4) under reflux of acetonitrile and MoCl(5) in dichloromethane at room temperature encouraged us to propose
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service