244988
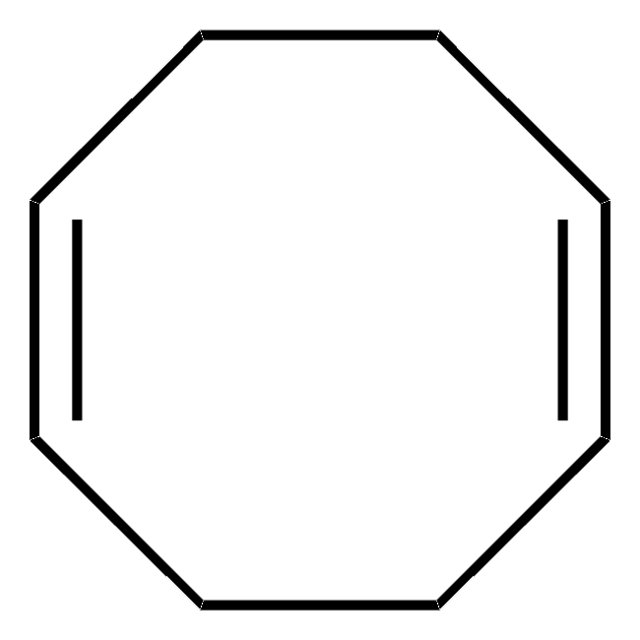
Bis(1,5-cyclooctadiene)nickel(0)
Synonym(s):
Bis(cyclooctadiene)nickel, Ni(COD)2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H24Ni
CAS Number:
Molecular Weight:
275.06
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
parameter
temperature sensitive
mp
60 °C (dec.) (lit.)
storage temp.
−20°C
SMILES string
[Ni].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.Ni/c2*1-2-4-6-8-7-5-3-1;/h2*1-2,7-8H,3-6H2;/b2*2-1-,8-7-;
InChI key
JRTIUDXYIUKIIE-KZUMESAESA-N
Application
Reactant for:
Catalyst for:
- Oxidative addition reactions
Catalyst for:
- Asymmetric α-arylation and heteroarylation of ketones with chloroarenes
- Cross-coupling reactions
- Regioselective and stereoselective carboxylation/cyclization of allenyl aldehydes under a carbon dioxide atmosphere
- Methyl carboxylation of homopropargylic alcohols
- Stereoselective borylative ketone-diene coupling
- Cycloaddition of benzamides with internal alkynes
Catalyst for the cycloaddition of 1,3-dienes.
Used to catalyze the addition of allyl phenyl sulfide to alkynes leading to 1,4-dienes. The reaction with terminal acetylenes proceeds in high yield and high selectivity. A variety of functional groups are tolerated.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Flam. Sol. 1 - Skin Sens. 1 - STOT RE 1
Target Organs
Lungs
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Eliad Benitez-Medina et al.
Dalton transactions (Cambridge, England : 2003), 48(47), 17579-17587 (2019-11-22)
The nickel-catalyzed N-alkylation of a variety of arylamines via transfer hydrogenation in the absence of pressurized hydrogen and basic or acidic additives was achieved in a tandem reaction. This process was further extended to the C[double bond, length as m-dash]N
Tiziana Funaioli et al.
PloS one, 5(5), e10617-e10617 (2010-05-21)
It is well known that, stemming from the mutual interplay between chromophores, circular dichroism (CD) is a powerful technique to deal with structural problems for both the small organic molecule and the biopolymer. However, quantitative interpretations of the spectroscopic and
Tsung-Han Lin et al.
ACS applied materials & interfaces, 9(5), 4948-4955 (2017-01-13)
The race for performance of integrated circuits is nowadays facing a downscale limitation. To overpass this nanoscale limit, modern transistors with complex geometries have flourished, allowing higher performance and energy efficiency. Accompanying this breakthrough, challenges toward high-performance devices have emerged
Joachim Loup et al.
Angewandte Chemie (International ed. in English), 58(6), 1749-1753 (2018-12-06)
Highly enantioselective nickel-catalyzed alkene endo-hydroarylations were accomplished with full selectivity by organometallic C-H activation. The asymmetric assembly of chiral six-membered scaffolds proved viable in the absence of pyrophoric organoaluminum reagents within an unprecedented nickel/JoSPOphos manifold.
Qiang Gao et al.
Acta biomaterialia, 51, 112-124 (2017-01-31)
Numerous antimicrobial coatings have been developed for biomedical devices/implants, but few can simultaneously fulfill the requirements for antimicrobial and antifouling ability and biocompatibility. In this study, to develop an antimicrobial and antibiofilm surface coating, diblock amphiphilic molecules with antimicrobial and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service