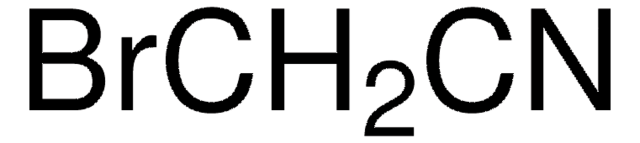225770
Lithium bis(trimethylsilyl)amide solution
1.0 M in THF
Synonym(s):
Hexamethyldisilazane lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
[(CH3)3Si]2NLi
CAS Number:
Molecular Weight:
167.33
Beilstein:
3567910
MDL number:
UNSPSC Code:
12352111
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
concentration
1.0 M in THF
density
0.891 g/mL at 25 °C
SMILES string
[Li]N([Si](C)(C)C)[Si](C)(C)C
InChI
1S/C6H18NSi2.Li/c1-8(2,3)7-9(4,5)6;/h1-6H3;/q-1;+1
InChI key
YNESATAKKCNGOF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Lithium bis(trimethylsilyl)amide (LHMDS) is a non-nucleophilic strong base widely used in organic synthesis for deprotonation reactions and base-catalyzed reactions. It is generally soluble in most nonpolar organic solvents.
Application
Lithium bis(trimethylsilyl)amide solution (1.0 M in THF) can be used as a base:
- To catalyze polymerization reaction in the synthesis of poly(p-benzamide)s.
- In the synthesis of cyclic poly(α-peptoid)s and α-(difluoromethyl)styrene.
- In directed aldol condensations and Darzens condensation reactions; α-arylation of aryl ester derivatives and allylic amination reaction.
- For the generation of kinetic enolates than sodium hexamethyldisilazide (NaHMDS) because the enolates produced are more regiostable than those produced with NaHMDS.
Packaging
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
Legal Information
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
recommended
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
? -(Difluoromethyl) styrene: improved approach to grams scale synthesis
Justyna WK,et al.
Journal of Fluorine Chemistry, 179, 175-178 (2015)
Macromolecules, 39, 5347-5347 (2006)
Juliana Tsz Yan Lee et al.
Scanning, 34(1), 12-25 (2012-04-26)
Common dehydration methods of cells on biomaterials for scanning electron microscopy (SEM) include air drying, hexamethyldisilazane (HMDS) or tetramethysilane (TMS) treatment and critical point drying (CPD). On the other side, freeze-drying has been widely employed in dehydrating biological samples and
Haoyu Tang et al.
Biomacromolecules, 11(6), 1585-1592 (2010-05-15)
A series of poly(gamma-chloropropyl-L-glutamate) (PCPLG) with controlled polymer molecular weight (MW = 5-28 kg x mol(-1)) and molecular weight distribution (PDI = 1.16-1.26) have been prepared from hexamethyldisilazane (HMDS)-mediated ring-opening polymerization (ROP) of gamma-chloropropyl-L-glutamic acid based N-carboxylanhydride (CP-NCA). CD, FTIR
Werner Mormann et al.
Macromolecular bioscience, 9(4), 369-375 (2008-11-26)
Trimethylsilylation of cellulose in different 1,3-dialkylimidazolium ionic liquids (IL) with hexamethyldisilazane (HMDS) as a silylating agent was investigated. Trimethylsilyl (TMSi) cellulose with a degree of substitution (DS) greater than 1 is insoluble in the IL. The maximum DS obtained depends
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service