155071
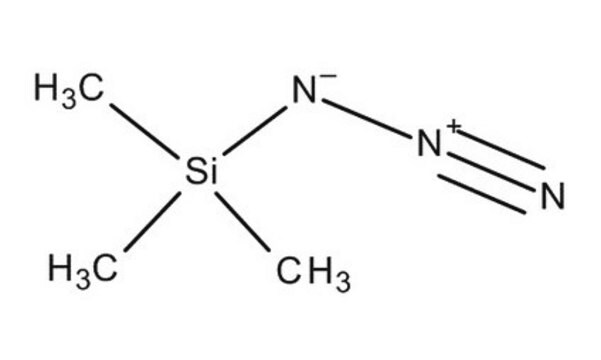
Azidotrimethylsilane
95%
Synonym(s):
Trimethylsilyl azide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3SiN3
CAS Number:
Molecular Weight:
115.21
Beilstein:
1903730
EC Number:
MDL number:
UNSPSC Code:
12352103
eCl@ss:
39100709
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
52-53 °C/175 mmHg (lit.)
density
0.868 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)N=[N+]=[N-]
InChI
1S/C3H9N3Si/c1-7(2,3)6-5-4/h1-3H3
InChI key
SEDZOYHHAIAQIW-UHFFFAOYSA-N
General description
Azidotrimethylsilane (TMSN3) is a a colorless and stable organosilane reagent. It shows very slow decomposition at high temperatures. It is a very commonly used azide source and has been used in the synthesis of aminotriazole ligands. Azidotrimethylsilane can be easily synthesised by adding chlorotrimethylsilane dropwise to a stirred solution of NaN3 in diethylene glycol dimethyl ether.
Application
Azidotrimethylsilane can be used as:
- A nitrogen precursor to prepare GaN nanowire via metal-organic chemical vapor deposition method.
- An electrolyte additive in Li-O2 batteries. The addition of TMSN3 results in the formation of robust solid electrolyte interphase.
- An efficient reagent in the synthesis of tetrazoles, fullerenyl azide, and α-azido oximes.
- A silylating agent in the O-trimethyl silylation of alcohols and phenols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Covalent functionalization of epitaxial graphene by azidotrimethylsilane
Choi, Junghun, et al.
The Journal of Physical Chemistry C, 113(22), 9433-9435 (2009)
Synthesis, 106-106 (1988)
Azidotrimethylsilane
Li, B. L.
Synlett, 23(10), 1554-1555 (2012)
Well-defined poly (oxazoline)-b-poly (acrylate) amphiphilic copolymers: From synthesis by polymer-polymer coupling to self-organization in water
Guillerm, Brieuc, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 51(5), 1118-1128 (2013)
James T Goettel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(5), 1136-1143 (2019-11-30)
A cyclic (alkyl)(amino)carbene (CAAC) has been shown to react with a covalent azide similar to the Staudinger reaction. The reaction of Me CAAC with trimethylsilyl azide afforded the N-silylated 2-iminopyrrolidine (Me CAAC=NSiMe3 ), which was fully characterized. This compound undergoes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service