145491
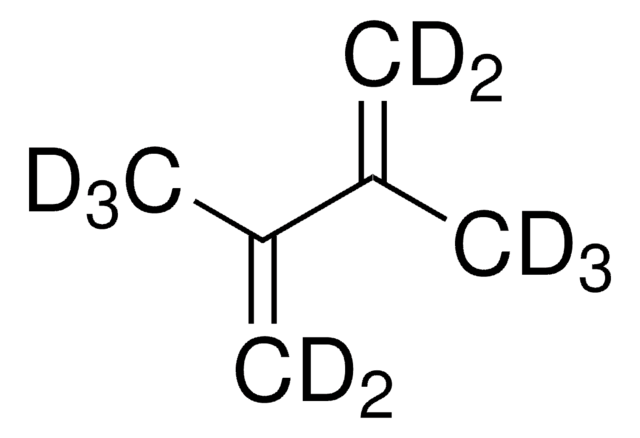
2,3-Dimethyl-1,3-butadiene
98%, contains 100 ppm BHT as stabilizer
Synonym(s):
2,3-Dimethylbuta-1,2-diene, 2,3-Dimethylenebutane, Biisopropenyl, Diisopropenyl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=C(CH3)C(CH3)=CH2
CAS Number:
Molecular Weight:
82.14
Beilstein:
605285
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
Assay:
98%
Recommended Products
vapor pressure
269 mmHg ( 37.7 °C)
Quality Level
Assay
98%
form
liquid
contains
100 ppm BHT as stabilizer
refractive index
n20/D 1.438 (lit.)
bp
68-69 °C (lit.)
mp
−76 °C (lit.)
density
0.726 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=C)C(C)=C
InChI
1S/C6H10/c1-5(2)6(3)4/h1,3H2,2,4H3
InChI key
SDJHPPZKZZWAKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3-Dimethyl-1,3-butadiene (DMBD) is a conjugated diene. It undergoes Diels Alder cycloaddition reaction with 2-thio-3-chloroacrylamides under thermal, catalytic and microwave conditions. It undergoes thermal [4+2] cycloaddition reaction with 3-acetyl-, 3-carbamoyl and 3-ethoxycarbonylcoumarins under solvent free conditions. DMBD participates in polymerization reactions in the presence of iron dichloride complexes based catalysts.
Application
2,3-Dimethyl-1,3-butadiene was used to investigate the reactions of 1,3-dienes with the Si(001) surface using scanning tunneling microscopy and fourier transform infrared spectroscopy.
It may be used in the following processes:
It may be used in the following processes:
- Preparation of 1,3,6-triene derivatives of corresponding 1-aryl-substituted 1,3-dienes by 1,4-hydrobutadienylation in the presence of cobalt catalyst.
- Synthesis of 6-aryl(hetaryl)-3,4-dimethyl-1-nitro-1-cyano-3-cyclohexenes by reacting with gem-cyanonitroethenes.
- As a halogen trap during the study of the photolysis reaction of dibromo adduct of 2,5-diphenyltellurophene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aryl (hetaryl)-containing gem-cyanonitroethenes: Synthesis, structure, and reactions with 2,3-dimethyl-1,3-butadiene.
Baichurin RI, et al.
Russ. J. Gen. Chem., 85(8), 1845-1854 (2015)
Cycloaddition chemistry of 1, 3-dienes on the silicon (001) surface: Competition between [4+ 2] and [2+ 2] reactions.
Hovis JS, et al.
The Journal of Physical Chemistry B, 102(35), 6873-6879 (1998)
Efficient halogen photoelimination from dibromo, dichloro and difluoro tellurophenes.
Carrera EI and Seferos DS.
Dalton Transactions, 44(5), 2092-2096 (2015)
Marie Kissane et al.
Organic & biomolecular chemistry, 8(24), 5602-5613 (2010-10-12)
The Diels-Alder cycloadditions of cyclopentadiene and 2,3-dimethyl-1,3-butadiene to a range of 2-thio-3-chloroacrylamides under thermal, catalytic and microwave conditions is described. The influence of reaction conditions on the outcome of the cycloadditions, in particular the stereoselectivity and reaction efficiency, is discussed.
Peroxy radical kinetics resulting from the OH-initiated oxidation of 1,3-butadiene, 2,3-dimethyl-1,3-butadiene and isoprene.
Jenkin ME, et al.
Journal of Atmospheric Chemistry, 29(3), 267-298 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service