All Photos(3)
About This Item
Empirical Formula (Hill Notation):
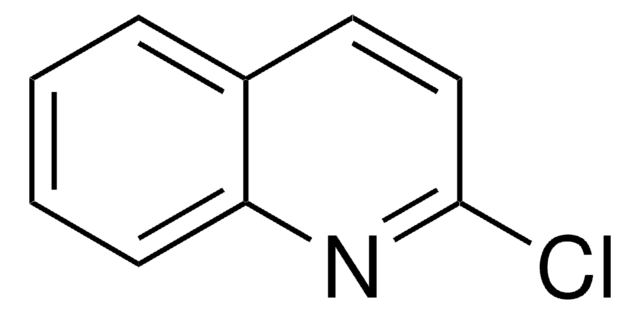
C8H5ClN2
CAS Number:
Molecular Weight:
164.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
100 °C/1.4 mmHg (lit.)
mp
47-50 °C (lit.)
functional group
chloro
SMILES string
Clc1cnc2ccccc2n1
InChI
1S/C8H5ClN2/c9-8-5-10-6-3-1-2-4-7(6)11-8/h1-5H
InChI key
BYHVGQHIAFURIL-UHFFFAOYSA-N
Application
2-Chloroquinoxaline (QCI) was used to study the effect of solvent on hydrolysis of QCI in aqueous–organic solvent mixtures with acetonitrile and dimethylesulphoxide. It was used in the synthesis of 2-(3-butynyl-2-methyl-2-ol) quinoxaline.It was used as reagent in the synthesis of chloroquinoxaline sulfamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetics of the Reaction of 2-Chloro-quinoxaline with Hydroxide Ion in ACN-H2O and DMSO-H2O Binary Solvent Mixtures.
Fathalla MF.
Journal of Solution Chemistry, 40(7), 1258-1270 (2011)
Kelly G Matz et al.
Inorganic chemistry, 50(20), 9804-9815 (2011-09-08)
A model system for the molybdenum cofactor has been developed that illustrates the noninnocent behavior of an N-heterocycle appended to a dithiolene chelate on molybdenum. The pyranopterin of the molybdenum cofactor is modeled by a quinoxalyldithiolene ligand (S(2)BMOQO) formed from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service