106399
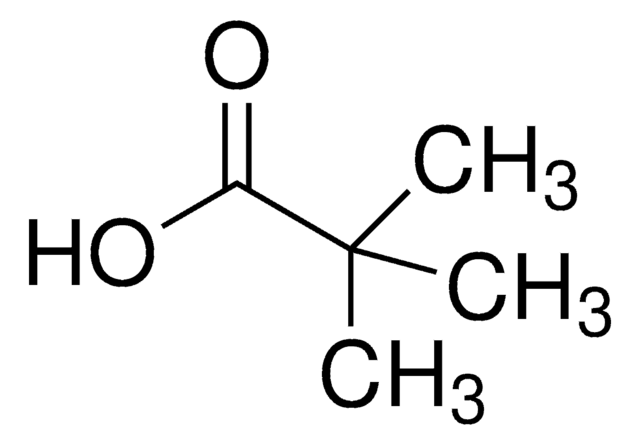
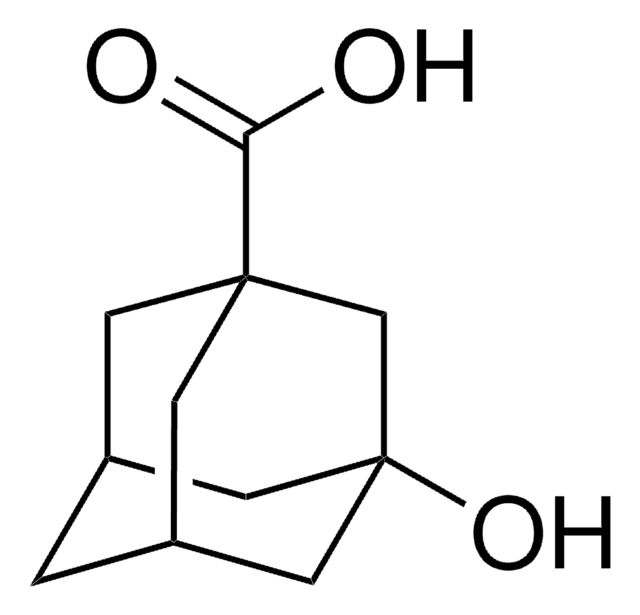
1-Adamantanecarboxylic acid
99%
Synonym(s):
Adamantane carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H16O2
CAS Number:
Molecular Weight:
180.24
Beilstein:
1910637
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Assay:
99%
Recommended Products
Quality Level
Assay
99%
mp
172-174 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C11H16O2/c12-10(13)11-4-7-1-8(5-11)3-9(2-7)6-11/h7-9H,1-6H2,(H,12,13)/t7-,8+,9-,11-
InChI key
JIMXXGFJRDUSRO-KJZNFTALSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-Adamantanecarboxylic acid can be used as:
- A stabilizer in the synthesis of monodisperse, highly crystalline CoPt3 nanoparticles and porous platinum nanoparticles.
- An additive in polycondensation reactions to yield conjugated polymers as possible optoelectronic materials.
- An additive in the allylic substitution reaction, which is catalyzed by palladium in an aqueous medium.
Biochem/physiol Actions
1-Adamantanecarboxylic acid undergoes complexation reactions with cyclohexaamylose. It is an inhibitor of phenyl ester hydrolysis of cycloheptaamylose.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Masaki Nakahata et al.
Nature communications, 2, 511-511 (2011-10-27)
Expanding the useful lifespan of materials is becoming highly desirable, and self-healing and self-repairing materials may become valuable commodities. The formation of supramolecular materials through host-guest interactions is a powerful method to create non-conventional materials. Here we report the formation
Structure of a complex of cycloheptaamylose with 1-adamantanecarboxylic acid.
Hamilton JA and Sabesan MN.
Acta Crystallographica Section B, Structural Crystallography and Crystal Chemistry, 38(12), 3063-3069 (1982)
Daniel Harries et al.
Journal of the American Chemical Society, 127(7), 2184-2190 (2005-02-17)
Using microcalorimetry, we follow changes in the association free energy of beta-cyclodextrin (CD) with the hydrophobic part of adamantane carboxylate (AD) due to added salt or polar (net-neutral) solutes that are excluded from the molecular interacting surfaces. Changes in binding
Birgit Hakkarainen et al.
Carbohydrate research, 340(8), 1539-1545 (2005-05-12)
The hydrogen-bond network in mono-altro-beta-cyclodextrin and in its inclusion complex with adamantane-1-carboxylic acid were investigated by (1)H NMR spectroscopy using the chemical shifts, temperature coefficients and vicinal coupling constants of the exchangeable hydroxy protons. The chemical shifts of the 3-OH
Detailed optimization of polycondensation reaction via direct C-H arylation of ethylenedioxythiophene
Yamazaki K, et al.
Macromolecular Rapid Communications, 34(1), 69-73 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service