E29109
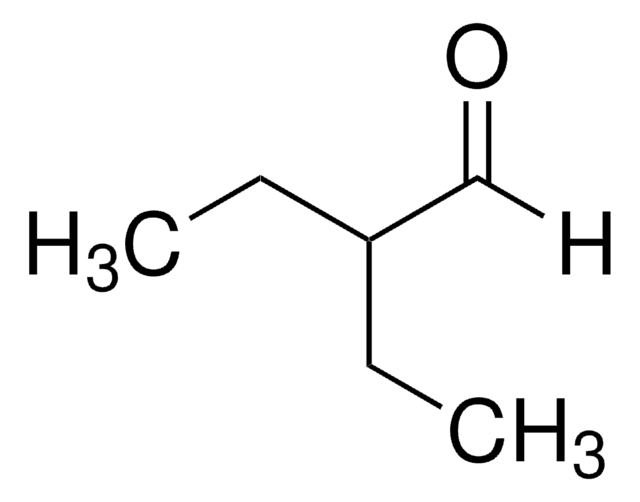
2-Ethylhexanal
96%
Synonym(s):
2-Ethylcapronaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3CH(C2H5)CHO
CAS Number:
Molecular Weight:
128.21
Beilstein:
1700556
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
impurities
<4% 2-ethylhexanoic acid
refractive index
n20/D 1.415 (lit.)
bp
55 °C/13.5 mmHg (lit.)
density
0.822 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)C(CC)CCCC
InChI
1S/C8H16O/c1-3-5-6-8(4-2)7-9/h7-8H,3-6H2,1-2H3
InChI key
LGYNIFWIKSEESD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Ethylhexanal is an aliphatic aldehyde used as an intermediate to produce various useful products such as perfumes, disinfectants, paints, warning agents, insecticides, and leak detectors.
Application
2-Ethylhexanal can be used as:
- A reductant in the Mukaiyama epoxidation of cis-cyclooctene.
- A reactant in the Horner-Wadsworth-Emmons reaction to synthesize cis-α,β-unsaturated amides.
- A starting material to synthesize 2-ethylhexanoic acid using manganese(II) acetate as a catalyst.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Repr. 2 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Continuous catalytic ?one-pot? multi-step synthesis of 2-ethylhexanal from crotonaldehyde
Seki T, et al.
Chemical Communications (Cambridge, England), 3562-3564 (2007)
Heather L Voegtle et al.
Journal of chemical ecology, 34(2), 215-219 (2008-01-24)
E-2-ethyl-2-hexen-1-ol (1), mellein (4), and 4-hydroxymellein (5) were identified as the major volatile compounds in the head and/or thorax of Camponotus quadrisectus. Neither 1 nor 5 have been previously detected in insects. Also identified were small amounts of m-cresol (2)
David M Hodgson et al.
The Journal of organic chemistry, 78(4), 1508-1518 (2013-01-30)
The synthesis and alkylation of chiral, nonracemic tropane- and homotropane-derived enamines is examined as an approach to enantioenriched α-alkylated aldehydes. The two bicyclic N auxiliaries, which differ by a single methylene group, give opposite senses of asymmetric induction on alkylation
Bis(2-t-butylphenyl) phosphonoacetamides for the highly cis-selective synthesis of α, β-unsaturated amides
Ando K, et al.
Tetrahedron Letters, 50, 5689-5691 (2009)
Factors affecting the selectivity of air oxidation of 2-ethyhexanal, an α-branched aliphatic aldehyde
Lehtinen C and Brunow G
Organic Process Research & Development, 4, 544-549 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service