D176605
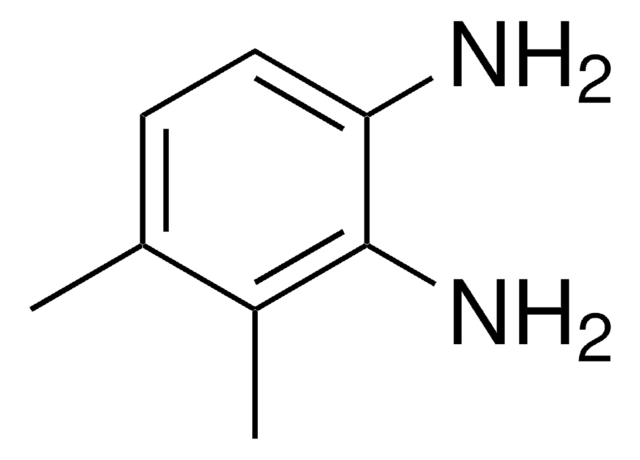
4,5-Dimethyl-1,2-phenylenediamine
98%
Synonym(s):
4,5-Diamino-o-xylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2C6H2(NH2)2
CAS Number:
Molecular Weight:
136.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
127-129 °C (lit.)
storage temp.
2-8°C
SMILES string
Cc1cc(N)c(N)cc1C
InChI
1S/C8H12N2/c1-5-3-7(9)8(10)4-6(5)2/h3-4H,9-10H2,1-2H3
InChI key
XSZYBMMYQCYIPC-UHFFFAOYSA-N
Application
Employed in the preparation of transition metal complexes and quinoxalinones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tijana Bugarcic et al.
Inorganic chemistry, 48(19), 9444-9453 (2009-09-29)
The synthesis and characterization of ruthenium(II) arene complexes of the general formula [(eta(6)-arene)Ru(XY)Z](+), where arene = p-cymene (p-cym), hexamethylbenzene (hmb), or biphenyl (bip), XY = o-phenylenediamine (o-pda), o-benzoquinonediimine (o-bqdi), or 4,5-dimethyl-o-phenylenediamine (dmpda), and Z = Cl, Br, or I, are
E Mikiciuk-Olasik et al.
Acta poloniae pharmaceutica, 51(3), 231-233 (1994-01-01)
Some of new derivatives of 4-acylquinoxalin-2-one obtained in the reaction of N,N'-bis(chloroacetyl)-4,5-dimethyl-o-phenylenediamine with primary amines are described. Compounds were tested for their anti-HIV activity and as ligands for 99mTc.
M L Karnovsky et al.
Environmental health perspectives, 102 Suppl 10, 43-44 (1994-12-01)
Although great progress has been made in understanding the respiratory burst of leukocytes that produce superoxide (O2-), it is possible that a component or components, might have been overlooked. Furthermore, O2- production and its sequels, though cardinal in bactericidal action
K Mori et al.
Chemical & pharmaceutical bulletin, 46(9), 1474-1476 (1998-10-17)
A simple and accurate method for determination of vitamin C (ascorbic acid (AsA) and dehydroascorbic acid (DHA)) using 4,5-dimethyl-o-phenylenediamine (DMPD) was investigated. It was found that DMPD is a useful fluorescent reagent. The reaction product of DMPD with DHA showed
M Katayama et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 110(10), 776-782 (1990-10-01)
Twelve purines oxidized with 4,5-dimethyl-o-phenylenediamine (DMPD) was found to react with N-bromosuccinimide (NBS) to give fluorescent derivatives. In this paper, the identification of oxidation and fluorescent products have been described. In compliance with the substituent on purine skelton, three alloxans
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service