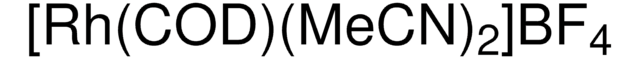695467
1,3,5-Triaza-7-phosphaadamantane
97%
Synonym(s):
1,3,5-Triaza-7-phosphatricyclo[3.3.1.13.7]decane, NSC 266642, PTA
About This Item
Recommended Products
Assay
97%
form
solid
reaction suitability
reagent type: ligand
reaction type: Hydroformylations
reagent type: ligand
reaction type: Hydrogenations
reagent type: ligand
reaction type: Morita-Baylis-Hillman Reactions
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
244-250 °C
functional group
phosphine
SMILES string
C1N2CN3CN1CP(C2)C3
InChI
1S/C6H12N3P/c1-7-2-9-3-8(1)5-10(4-7)6-9/h1-6H2
InChI key
FXXRPTKTLVHPAR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- The molecular mechanisms of antimetastatic ruthenium compounds explored through DIGE proteomics.: This study examines the antimetastatic properties of ruthenium compounds using DIGE proteomics. The involvement of 1,3,5-Triaza-7-phosphaadamantane in the complexation with ruthenium and its biological effects were analyzed, highlighting its potential in anticancer therapies (Guidi et al., 2013).
- Synthesis, antimicrobial and antiproliferative activity of novel silver(I) tris(pyrazolyl)methanesulfonate and 1,3,5-triaza-7-phosphadamantane complexes.: This research details the synthesis of novel silver complexes containing 1,3,5-Triaza-7-phosphaadamantane, evaluating their antimicrobial and antiproliferative activities, which demonstrates the compound′s utility in biomedical applications (Pettinari et al., 2011).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Related Content
The Frost group has a longstanding interest in aqueous phase organometallic chemistry with an emphasis on water-soluble phosphine ligands. One focus of this research group has been centered on the chemistry of the neutral, air stable, and water-soluble heterocyclic phosphine 1,3,5-Triaza-7-phosphaadamantane (PTA). PTA is a small phosphine ligands with a cone angle of ~103°. PTA binds metal centers quite strongly and is electronically more donating than PPh3. Ruthenium complexes of PTA have exhibited impressive anticancer activity. A wide variety of transition metal complexes of PTA have been utilized as catalysts for reactions such as hydrogenation, C-C bond forming reactions, atom transfer radical addition, and nitrile hydration.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
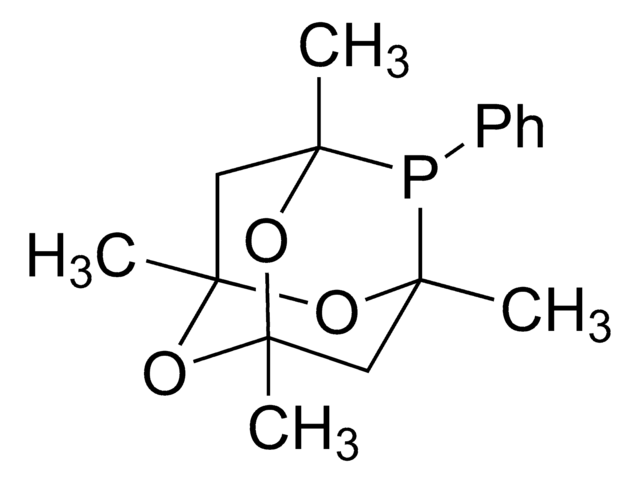
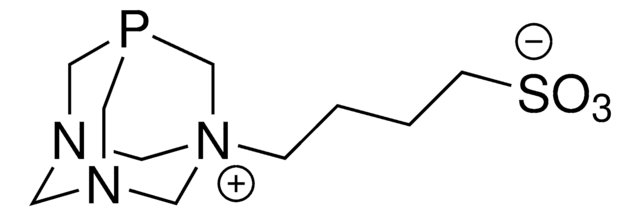
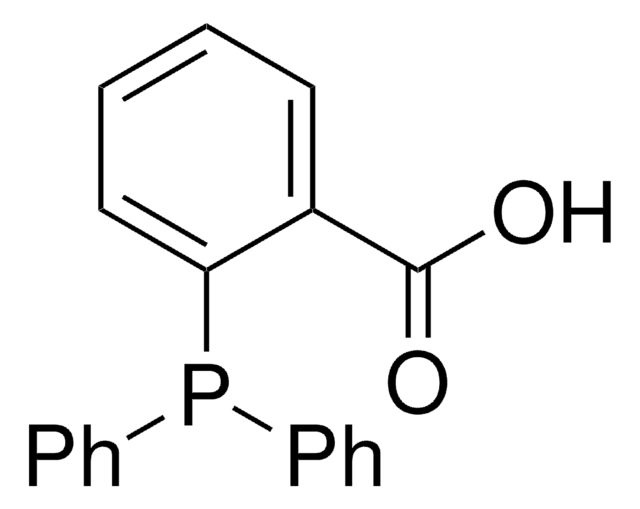

![3,7-Diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane 97%](/deepweb/assets/sigmaaldrich/product/structures/198/979/42d0b946-b026-4831-b284-fcb0e91533d9/640/42d0b946-b026-4831-b284-fcb0e91533d9.png)
![1,4-Bis[(phenyl-3-propanesulfonate) phosphine] butane disodium salt](/deepweb/assets/sigmaaldrich/product/structures/322/102/cc3c448f-049a-41a4-93c3-26d70302d06d/640/cc3c448f-049a-41a4-93c3-26d70302d06d.png)


![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)
![2,8,9-Triisobutyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane 97%](/deepweb/assets/sigmaaldrich/product/structures/750/287/cc77a98e-fa6c-4d81-9f3e-f392770724ac/640/cc77a98e-fa6c-4d81-9f3e-f392770724ac.png)