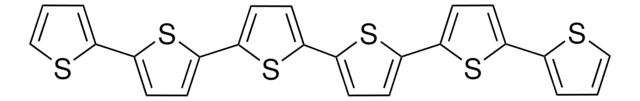
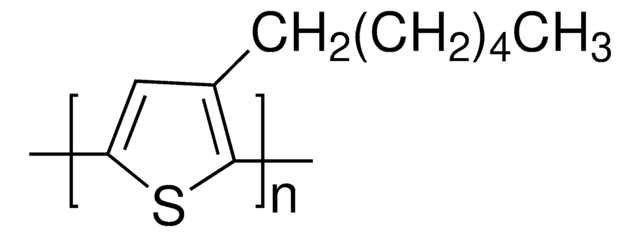
525936
Poly(thiophene-2,5-diyl), bromine terminated
powder
Synonym(s):
Thiophene, polymers
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
powder
Quality Level
mp
>350 °C
InChI
1S/C4H4S/c1-2-4-5-3-1/h1-4H
InChI key
YTPLMLYBLZKORZ-UHFFFAOYSA-N
Related Categories
General description
Conducting polymer.
Packaging
Packaged in glass bottles
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yi Huang et al.
Biosensors & bioelectronics, 44, 41-47 (2013-02-09)
We report here on a facile enzymatic polymerization protocol to prepare enzyme-poly(thiophene-3-boronic acid) (PTBA) polymeric biocomposites (PBCs) for high-performance mono-/bi-enzyme amperometric biosensing. Horseradish peroxidase (HRP)-catalyzed polymerization of thiophene-3-boronic acid (TBA) monomer was conducted in aqueous solution containing HRP (or plus
Rajesh Kumar et al.
Journal of the American Chemical Society, 134(36), 14869-14876 (2012-08-04)
A three terminal molecular memory device was monitored with in situ Raman spectroscopy during bias-induced switching between two metastable states having different conductivity. The device structure is similar to that of a polythiophene field effect transistor, but ethylviologen perchlorate was
Hiroyuki Tamura et al.
The Journal of chemical physics, 137(22), 22A540-22A540 (2012-12-20)
Following up on our recent study of ultrafast charge separation at oligothiophene-fullerene interfaces [H. Tamura, I. Burghardt, and M. Tsukada, J. Phys. Chem. C 115, 10205 (2011)], we present here a detailed quantum dynamical perspective on the charge transfer process.
C Zanardi et al.
Analytical and bioanalytical chemistry, 405(2-3), 509-531 (2012-09-04)
This overview of polythiophene-based materials provides a critical examination of meaningful examples of applications of similar electrode materials in electroanalysis. The advantages arising from the use of polythiophene derivatives in such an applicative context is discussed by considering the organic
Dmytro Dudenko et al.
Angewandte Chemie (International ed. in English), 51(44), 11068-11072 (2012-10-06)
To tilt or not to tilt: The crystal structure for bulk P3HT (phase I) was determined by "multi-technique crystallography", which combines X-ray diffraction, solid-state NMR spectroscopy, and DFT calculations. The results showed that this semiconducting polymer crystallizes in the monoclinic space
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service