All Photos(1)
About This Item
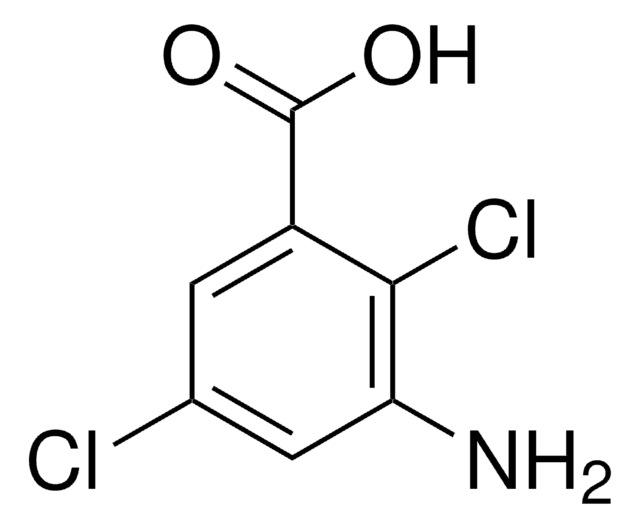
Linear Formula:
H2NC6H3(Cl)CO2H
CAS Number:
Molecular Weight:
171.58
Beilstein:
2803668
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: solution phase peptide synthesis
mp
211 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
Nc1ccc(C(O)=O)c(Cl)c1
InChI
1S/C7H6ClNO2/c8-6-3-4(9)1-2-5(6)7(10)11/h1-3H,9H2,(H,10,11)
InChI key
MBDUKNCPOPMRJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G Menon et al.
Journal of pharmaceutical sciences, 73(2), 251-253 (1984-02-01)
A high-performance liquid chromatographic method has been developed for the simultaneous determination of chloroprocaine hydrochloride and its hydrolytic degradation product, 4-amino-2-chlorobenzoic acid. Separation is achieved using a mu-Bondapak C18 column and the eluant, water-acetonitrile-methanol-glacial acetic acid (74:20:5:1) containing 0.05-0.08% (w/v)
F Brun et al.
Journal of pharmaceutical and biomedical analysis, 14(8-10), 1251-1259 (1996-06-01)
The separation of HPLC of basic drugs on silica-based reversed phases remains a major problem because of the interaction between the residual silanol groups of the silica and the amino function of the drug. This paper describes the validation of
E H Philipson et al.
American journal of obstetrics and gynecology, 146(1), 16-22 (1983-05-01)
Most of the reports of fetal bradycardia and acidosis following paracervical block anesthesia have involved the use of amide-linked anesthetics, such as lidocaine and mepivacaine. The purposes of this study were (1) to determine placental transfer of an ester-linked local
K Krohg et al.
Anesthesiology, 54(4), 329-332 (1981-04-01)
The purpose of this study was to identify the metabolic pathway of 2-chloro-4-aminobenzoic acid (CABA), a primary metabolite of chloroprocaine. Urine was collected from 4 healthy, pregnant women following the epidural administration of 600 mg chloroprocaine. The urinary metabolites were
P K Janicki et al.
Journal of chromatography. B, Biomedical applications, 675(2), 336-341 (1996-01-26)
A sensitive and specific high-performance liquid chromatographic method for determination of the 2-chloroprocaine, local anesthetic of ester type, and its major metabolite 2-chloroaminobenzoic acid, has been developed and validated. A single-step extraction procedure is employed followed by high-performance liquid chromatographic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service