216690
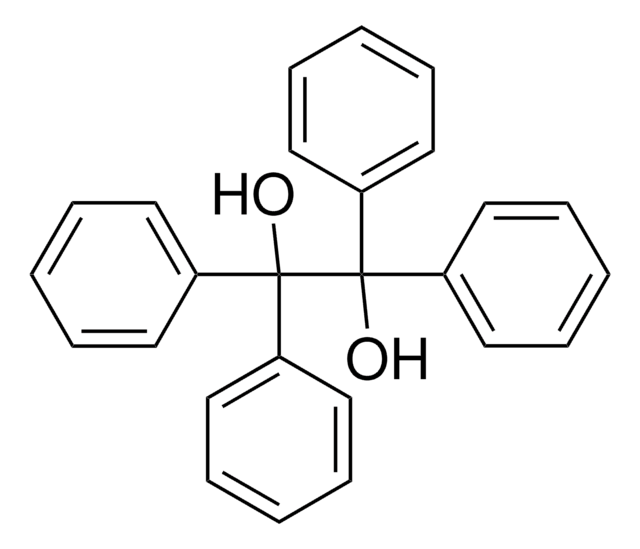
2,2,2-Triphenylacetophenone
98%
Synonym(s):
Benzopinacolone, Phenyl trityl ketone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C6H5)3CCOC6H5
CAS Number:
Molecular Weight:
348.44
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
182-184 °C (lit.)
functional group
ketone
SMILES string
O=C(c1ccccc1)C(c2ccccc2)(c3ccccc3)c4ccccc4
InChI
1S/C26H20O/c27-25(21-13-5-1-6-14-21)26(22-15-7-2-8-16-22,23-17-9-3-10-18-23)24-19-11-4-12-20-24/h1-20H
InChI key
CFBBKHROQRFCNZ-UHFFFAOYSA-N
General description
Asymmetric reduction of 2,2,2-triphenylacetophenone using with potassium 9-O-(1,2: 5, 6-di-O-isopropylidene-α-D-glucofuranosyl)-9-boratabicyclo [3.3.1] nonane (chiral reducing agent) has been reported. Dehydrative cyclization of 2,2,2-triphenylacetophenone has been reported.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Douglas A Klumpp et al.
Applied catalysis. A, General, 336(1-2), 128-132 (2008-03-01)
The hydroxyalkylation reactions of aceanthrenequinone (6) and acenapthenequinone (7) with a series of arenes have been studied. In reactions with the Brønsted superacid CF(3)SO(3)H (triflic acid), the condensation products are formed in good yields (58-99%, 10 examples) with high regioselectivity.
Chiral synthesis via organoboranes. 15. Selective reductions. 42. Asymmetric reduction of representative prochiral ketones with potassium 9-O-(1, 2: 5, 6-di-O-isopropylidene-. alpha.-D-glucofuranosyl)-9-boratabicyclo [3.3. 1] nonane.
Brown HC, etal.
The Journal of Organic Chemistry, 53(6), 1231-1238 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service