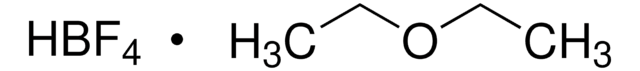207934
Tetrafluoroboric acid solution
48 wt. % in H2O
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
HBF4
Número de CAS:
Peso molecular:
87.81
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.21
concentration:
46.0-52.0% in NaOH (titration)
48 wt. % in H2O
48 wt. % in H2O
Productos recomendados
vapor density
3 (vs air)
Quality Level
vapor pressure
5 mmHg ( 20 °C)
form
liquid
concentration
46.0-52.0% in NaOH (titration)
48 wt. % in H2O
density
1.4 g/mL at 25 °C
SMILES string
F.FB(F)F
InChI
1S/BF3.FH/c2-1(3)4;/h;1H
InChI key
LEMQFBIYMVUIIG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Tetrafluoroboric acid is used as a catalyst for the protection and deprotection reactions of various carbohydrates. It participates in the synthesis of 4-sulfonic acid phenyl diazonium tetrafluoroborate, which was required for the preparation of sulfonated graphene (SG).
Application
Tetrafluoroboric acid solution may be used as a catalyst for the hydration of aromatic haloalkynes to α-halomethyl ketones in the absence of metal catalysts. It may also be used the epoxidized soybean oil (ESBO) ring opening step of fatty acids preparation.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Tetrafluoroboric acid, an efficient catalyst in carbohydrate protection and deprotection reactions.
Albert R, et al.
Carbohydrate Research, 137, 282-290 (1985)
Polyols and polyurethanes prepared from epoxidized soybean oil ring-opened by polyhydroxy fatty acids with varying OH numbers.
Chen R, et al.
Journal of Applied Polymer Science, 132(1) (2015)
M P Dariel et al.
Dental materials : official publication of the Academy of Dental Materials, 11(3), 208-217 (1995-05-01)
The purpose of this study was to develop a new technology for preparing mercury-free metallic dental restorative materials. The novel approach relies on the cold welding of surface treated silver particles. At ambient temperature, intermetallic compound formation takes place spontaneously
Arnis Abolins et al.
Materials (Basel, Switzerland), 14(4) (2021-03-07)
A second-generation bio-based feedstock-tall oil fatty acids-was epoxidised via two pathways. Oxirane rings were introduced into the fatty acid carbon backbone using a heterogeneous epoxidation catalyst-ion exchange resin Amberlite IR-120 H or enzyme catalyst Candida antarctica lipase B under the
Gary A Molander et al.
Organic letters, 12(21), 4876-4879 (2010-10-01)
Amidomethyltrifluoroborates were successfully synthesized in a one-pot fashion and used in cross-coupling reactions with a wide variety of aryl and heteroaryl chlorides.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico