M51407
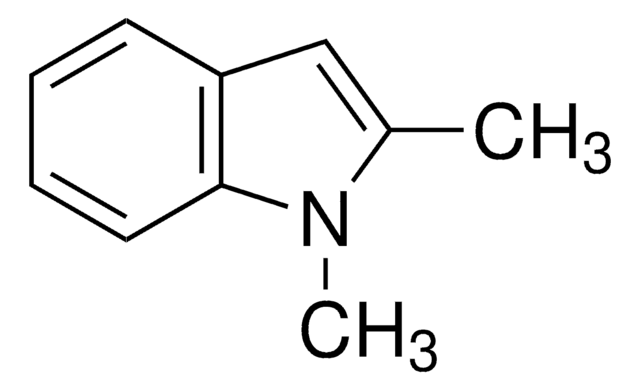
2-Methylindole
98%
Sinónimos:
NSC 7514
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H9N
Número de CAS:
Peso molecular:
131.17
Beilstein:
109781
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
98%
bp
273 °C (lit.)
mp
57-59 °C (lit.)
cadena SMILES
Cc1cc2ccccc2[nH]1
InChI
1S/C9H9N/c1-7-6-8-4-2-3-5-9(8)10-7/h2-6,10H,1H3
Clave InChI
BHNHHSOHWZKFOX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Reactant for:
- Regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition reaction
- Friedel-Crafts alkylation reactions
- Preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Preparation of plant-growth inhibitors
- Michael addition reactions
- Synthesis of cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
285.8 °F
Punto de inflamabilidad (°C)
141 °C
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Emma L Harry et al.
The Analyst, 136(8), 1728-1732 (2011-02-26)
The potential of ion mobility (IM) spectrometry in combination with mass spectrometry (MS) for real-time reaction monitoring is reported. The combined IM-MS approach using electrospray ionization affords gas-phase analyte characterization based on both mass-to-charge (m/z) ratio and gas-phase ion mobility
T Misra et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(8), 1631-1641 (2002-08-09)
By using steady state and time-resolved (laser flash photolysis and single photon counting) spectroscopic techniques the quenching of the lowest excited singlet (S1) state of 9-cyanoanthracene (9CNA) by the donors (quenchers) 2-methylindole (2MI) and 2-methylindoline (2MIN) in solvents of different
T Bhattacharya et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 60(8-9), 1957-1966 (2004-07-14)
Electrochemical measurements by cyclic voltammetry predict the possibility of occurrence of photoinduced electron-transfer (PET) reactions between the ground state of 2-phenylindole (2PI) (electron donor) and the excited singlet of 9-cyanoanthracene (9CNA) molecule acting as an electron acceptor. However, 2PI should
J M Gutteridge
The International journal of biochemistry, 14(7), 649-653 (1982-01-01)
1. The thiobarbituric acid (TBA) reaction, widely applied to the detection of autoxidation in polyunsaturated fatty acids, can be used to measure free-radical damage to amino acids, carbohydrates and nucleic acids. 2. In all of these systems malondialdehyde (MDA) is
V F Ximenes et al.
Archives of biochemistry and biophysics, 387(2), 173-179 (2001-05-24)
The indole moeity is present in many substances of biological occurrence. Its metabolism, in most cases, involves an oxidative pathway. This study reports the oxidation of a series of indole derivatives, including several of biological origin, catalyzed by horseradish peroxidase
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico